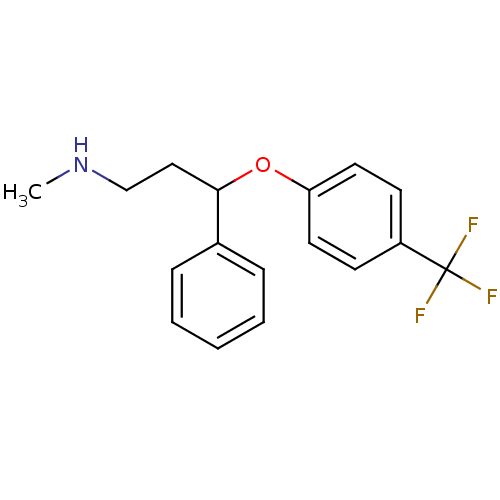
BDBM30130 CHEMBL1201082::CHEMBL41::Fluoxetin::Fluoxetine::Prozac::US9120771, Fluoxetine::cid_62857
SMILES CNCCC(Oc1ccc(cc1)C(F)(F)F)c1ccccc1
InChI Key InChIKey=RTHCYVBBDHJXIQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 263 hits for monomerid = 30130
Found 263 hits for monomerid = 30130
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 0.720nMAssay Description:Binding inhibition towards human serotonin transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.10nM ΔG°: -12.1kcal/mole EC50: 7.30nMpH: 7.4 T: 2°CAssay Description:Binding affinity of each compound was measured by assessing the potency of inhibition of binding of radiolabeled RTI-55. Membranes were preincubated ...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]Citalopram from human SERT by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [125I]RTI55 from human recombinant SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2nMAssay Description:Displacement of [3H]citalopram from serotonin transporter in Sprague-Dawley rat brain after 1 hr by liquid scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Binding affinity at human SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 2.20nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.70nMAssay Description:Displacement of [3H]paroxetine from 5HT transporter in rat cortical membraneMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.70nMAssay Description:Displacement of [3H]paroxetine from rat cortical 5HTT reuptake siteMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.72nMAssay Description:Binding affinity against serotonin transporter in rat cortical tissues using radioligand [3H]-paroxetineMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.80nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.90nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.90nMAssay Description:Binding affinity to rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 2.90nMAssay Description:Binding affinity at rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.17nMAssay Description:Evaluated for affinity at 5-HT uptake site using [3H]paroxetine as radioligand in radioligand binding assayMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.5nMAssay Description:Compound was evaluated for its binding affinity towards human serotonin transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]paroxetine from serotonin transporter in Sprague-Dawley rat frontal cortical membraneMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 3.90nMAssay Description:Displacement of [3H]paroxetine from 5-HT transporter in Sprague-Dawley rat cortical membranesMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 5.60nMAssay Description:Displacement of [3H]citalopram from SERT in Sprague-Dawley rat whole brain membranes after 1 hr by liquid scintillation spectrophotometric analysisMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetAlpha-2A adrenergic receptor(Homo sapiens (Human))
Institut De Recherches Servier
Curated by PDSP Ki Database
Institut De Recherches Servier
Curated by PDSP Ki Database
Affinity DataKi: 6.30nMAssay Description:Binding affinity for human alpha-2 adrenergic receptor expressed in CHO cellMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 7.30nMAssay Description:Inhibition of high affinity re-uptake of [3H]5-HT (serotonin) into nerve ending synaptosomesMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 7.30nMAssay Description:Inhibition of [3H]5-HT uptake by Serotonin transporter of rat midbrain or parietal synaptosomesMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 7.30nMAssay Description:Inhibition of [3H]-5-HT uptake into rat synaptosomes by Serotonin transporterMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 8.70nMAssay Description:Displacement of [3H]citalopram from Sprague-Dawley rat SERTMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 10nMAssay Description:In vitro binding affinity was determined against serotonin reuptake site of rat in presence of [3H]paroxetine radioligandMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 11nMAssay Description:Tested in vitro for serotonin(5-HT) neuronal uptake inhibitionMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 12.2nMAssay Description:Inhibition of SERT in rat cerebral cortex assessed as [3H]serotonin accumulationMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 14nMAssay Description:Inhibition of [3H]5-HT reuptake into rat frontal cortex synaptosomesMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 22nMAssay Description:Displacement of [3H]citalopram from rat cortical serotonin transporter (SERT)More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rattus norvegicus (rat))
Veterans Administration Medical Center
Curated by PDSP Ki Database
Veterans Administration Medical Center
Curated by PDSP Ki Database
Affinity DataKi: 22nMAssay Description:Inhibition of [3H]citalopram binding to Serotonin transporter of rat cerebral cortexMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Rattus norvegicus (Rat))
Roche Bioscience
Curated by PDSP Ki Database
Roche Bioscience
Curated by PDSP Ki Database
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Displacement of [3H]citalopram from human SERT in HEK293 cells by Topcount scintillation analysisMore data for this Ligand-Target Pair