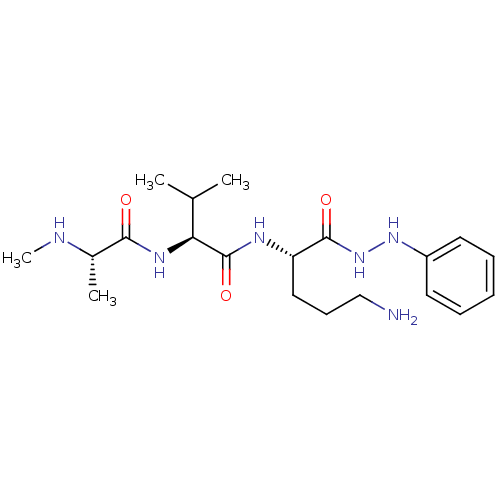
BDBM44328 (2S)-N-[(1S)-4-amino-1-(anilinocarbamoyl)butyl]-3-methyl-2-[[(2S)-2-(methylamino)propanoyl]amino]butyramide;formic acid::(2S)-N-[(2S)-5-amino-1-oxo-1-(2-phenylhydrazinyl)pentan-2-yl]-3-methyl-2-[[(2S)-2-(methylamino)propanoyl]amino]butanamide;formic acid::(2S)-N-[(2S)-5-amino-1-oxo-1-(phenylhydrazo)pentan-2-yl]-3-methyl-2-[[(2S)-2-(methylamino)-1-oxopropyl]amino]butanamide;formic acid::(2S)-N-[(2S)-5-azanyl-1-oxidanylidene-1-(2-phenylhydrazinyl)pentan-2-yl]-3-methyl-2-[[(2S)-2-(methylamino)propanoyl]amino]butanamide;methanoic acid::CHEMBL1623606::MLS-0412177.0001::cid_44182249
SMILES CN[C@@H](C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CCCN)C(=O)NNc1ccccc1
InChI Key InChIKey=KGALEDDRBNKPDD-XIRDDKMYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 44328
Found 3 hits for monomerid = 44328
TargetE3 ubiquitin-protein ligase XIAP(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 1.31E+4nMAssay Description:Competitive inhibition of XIAP-BIR2 domain (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetE3 ubiquitin-protein ligase XIAP(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataKi: 3.90E+4nMAssay Description:Competitive inhibition of XIAP-BIR3 domain (unknown origin) by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetE3 ubiquitin-protein ligase XIAP(Homo sapiens (Human))
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.03E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair