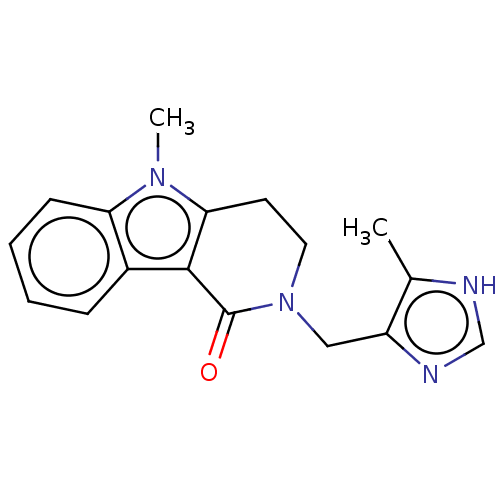
BDBM50014558 ALOSETRON::CHEBI:253342::Lotronex::US9045501, Alosetron
SMILES Cc1[nH]cnc1CN1CCc2c(C1=O)c1ccccc1n2C
InChI Key InChIKey=JSWZEAMFRNKZNL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50014558
Found 3 hits for monomerid = 50014558
Affinity DataKi: 0.5nM ΔG°: -12.7kcal/molepH: 7.5 T: 2°CAssay Description:The relative affinity of the various compounds for the human 5-HT3 receptor was measured in a radioligand binding assay, using a scintillation proxim...More data for this Ligand-Target Pair
Affinity DataKi: 0.5nMAssay Description:Displacement of [9-methyl-3H]BRL-43694 from human 5-HT3A receptor after overnight incubation by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair