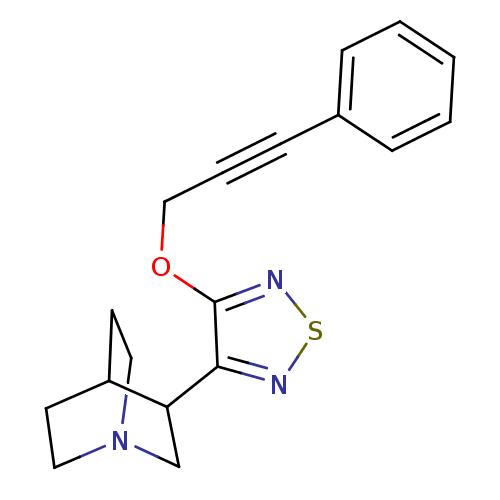
BDBM50072228 3-[4-(3-Phenyl-prop-2-ynyloxy)-[1,2,5]thiadiazol-3-yl]-1-aza-bicyclo[2.2.2]octane::CHEMBL99521::NNC 11-1314
SMILES C(Oc1nsnc1C1CN2CCC1CC2)C#Cc1ccccc1
InChI Key InChIKey=SNSMIIQEKVYNOH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50072228
Found 8 hits for monomerid = 50072228
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M4(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
TargetMuscarinic acetylcholine receptor M3(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
Affinity DataIC50: 4.70nMAssay Description:Binding affinity against muscarinic receptor in rat brain membranes using oxotremorine-M as ligandMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M1(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
Affinity DataEC50: 16nMAssay Description:Stimulation of phosphoinositide hydrolysis in A9L cells expressing human m1 receptorMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M2(Homo sapiens (Human))
University of Melbourne
Curated by PDSP Ki Database
University of Melbourne
Curated by PDSP Ki Database
Affinity DataEC50: 62nMAssay Description:Stimulation of cAMP in CHO cells expressing human m2 receptorMore data for this Ligand-Target Pair