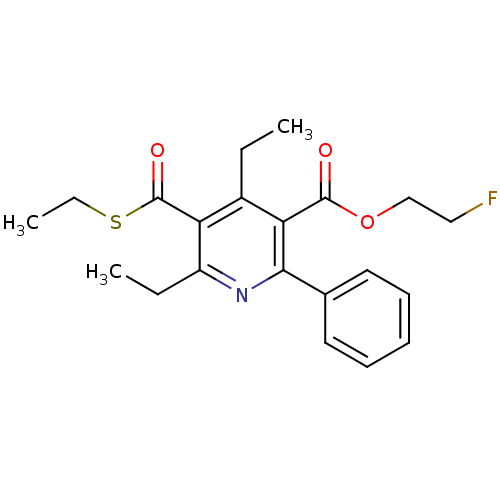
BDBM50074220 4,6-Diethyl-5-ethylsulfanylcarbonyl-2-phenyl-nicotinic acid 2-fluoro-ethyl ester::CHEMBL164321
SMILES CCSC(=O)c1c(CC)nc(-c2ccccc2)c(C(=O)OCCF)c1CC
InChI Key InChIKey=ZHOWYLFZKMSPTC-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50074220
Found 4 hits for monomerid = 50074220
Affinity DataKi: 4.22nMAssay Description:Displacement of specific [125 I]AB-MECA binding at human Adenosine A3 receptor expressed in HEK cellsMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Displacement of specific [125I]-AB-MECA binding at rat Adenosine A3 receptor stably expressed in CHO cellsMore data for this Ligand-Target Pair
TargetAdenosine receptor A2a(Rattus norvegicus (rat))
National Institute Of Diabetes
Curated by ChEMBL
National Institute Of Diabetes
Curated by ChEMBL
Affinity DataKi: 7.33E+3nMAssay Description:Inhibition of [3H]-CGS- 21680 binding to Adenosine A2A receptor in rat striatal membranes.More data for this Ligand-Target Pair
Affinity DataKi: 1.15E+4nMAssay Description:Displacement of specific [3H]-CGS- 21680R-PIA binding to Adenosine A1 receptor in rat brain membranes.More data for this Ligand-Target Pair