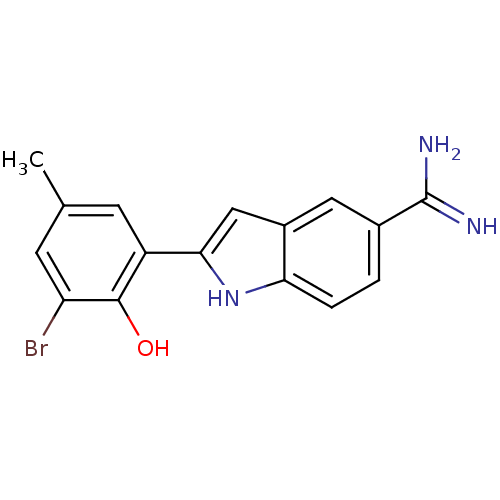
BDBM50101873 2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-carboxamidine::2-{5-[AMINO(IMINIO)METHYL]-1H-INDOL-2-YL}-6-BROMO-4-METHYLBENZENOLATE::CHEMBL48608
SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N
InChI Key InChIKey=BVTBOJXEAPSOEB-UHFFFAOYSA-N
Data 11 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50101873
Found 11 hits for monomerid = 50101873
Affinity DataKi: 52nMAssay Description:Binding affinity against human coagulation factor XMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 55nMAssay Description:ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA).More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity against Coagulation factor X in human plasmaMore data for this Ligand-Target Pair
TargetUrokinase-type plasminogen activator(Homo sapiens (Human))
Axys Pharmaceuticals
Curated by ChEMBL
Axys Pharmaceuticals
Curated by ChEMBL
Affinity DataKi: 110nMAssay Description:Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Inhibition of Human Serine Protease Thrombin.More data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Inhibition of Human Serine Protease Trypsin.More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Inhibitory activity against thrombin(fIIa) in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 460nMAssay Description:Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA).More data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Inhibition of Human Serine Protease Plasmin.More data for this Ligand-Target Pair
Affinity DataKi: 640nMAssay Description:Inhibitory activity against trypsin in human plasmaMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Inhibitory activity against plasmin in human plasmaMore data for this Ligand-Target Pair