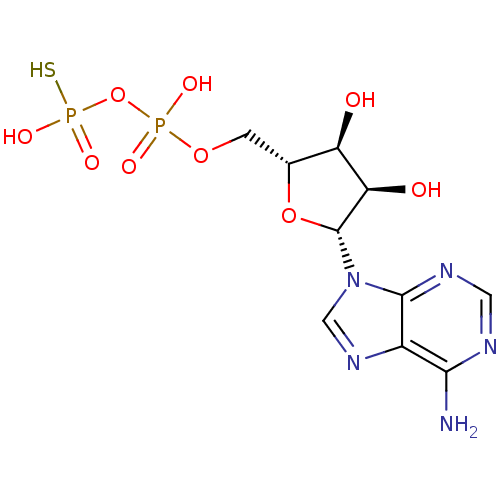
BDBM50118230 5'-O-[(R)-HYDROXY(THIOPHOSPHONOOXY)PHOSPHORYL]ADENOSINE::ADPbetaS::CHEMBL335206::adenosine-5'-O-beta-thiodiphosphate
SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)(S)=O)[C@@H](O)[C@H]1O
InChI Key InChIKey=HCIKUKNAJRJFOW-KQYNXXCUSA-N
Data 9 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50118230
Found 9 hits for monomerid = 50118230
Affinity DataEC50: 96nMAssay Description:Evaluated for agonist activity against phospholipase C coupled P2Y purinoceptor 1 (P2Y1) of turkey erythrocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 3.27E+4nMAssay Description:Agonist activity at GFP-tagged human P2Y11R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-bas...More data for this Ligand-Target Pair
Affinity DataEC50: 1.27E+3nMAssay Description:Agonist activity at GFP-tagged human P2Y1R transfected in human 1321N1 cells assessed as increase in intracellular Ca2+ level by fura 2/AM probe-base...More data for this Ligand-Target Pair
Affinity DataEC50: 2.50E+3nMAssay Description:The compound was evaluated for antagonist activity against recombinant human P2X purinoceptor 1 (P2X1 )More data for this Ligand-Target Pair
Affinity DataEC50: 1.00E+5nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat receptor P2X purinoceptor 2 (P2X2) at 10 uM, expressed in Xenopus oocytesMore data for this Ligand-Target Pair
Affinity DataEC50: 82nMAssay Description:Antagonist activity against phospholipase C coupled rat P2Y purinoceptor 12 (P2Y12)More data for this Ligand-Target Pair
Affinity DataEC50: 2.50E+4nMAssay Description:Antagonist activity against recombinant human P2X purinoceptor 4 (P2X4)More data for this Ligand-Target Pair
Affinity DataEC50: 3.00E+4nMAssay Description:The compound was evaluated for antagonist activity against phospholipase C coupled human P2Y purinoceptor 11 (P2Y11)More data for this Ligand-Target Pair
Affinity DataEC50: 8.80E+3nMAssay Description:The compound was evaluated for antagonist activity against recombinant rat P2X purinoceptor 3 (P2X3) at 10 uM, expressed in Xenopus oocytesMore data for this Ligand-Target Pair