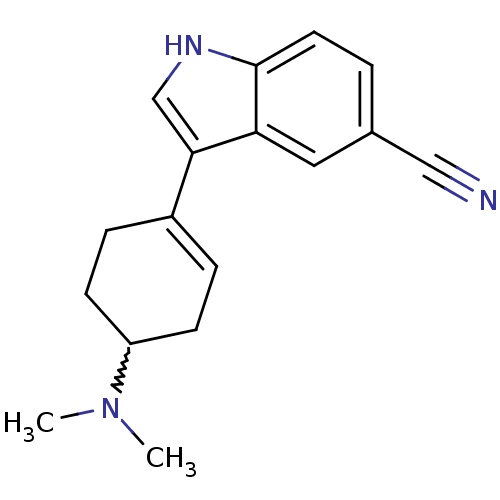
BDBM50209166 3-(4-(dimethylamino)cyclohex-1-enyl)-1H-indole-5-carbonitrile::CHEMBL390432
SMILES CN(C)C1CCC(=CC1)c1c[nH]c2ccc(cc12)C#N
InChI Key InChIKey=RSNYUBDIPFPZKJ-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50209166
Found 4 hits for monomerid = 50209166
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.340nMAssay Description:Displacement of [125I]RTI-55 from human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent noradrenaline transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 57nMAssay Description:Displacement of [125I]Nisoxetine from human NET expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Displacement of [3H]citalopram from human SERT expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Displacement of [125I]RTI-55 from human DAT expressed in HEK293 cellsMore data for this Ligand-Target Pair