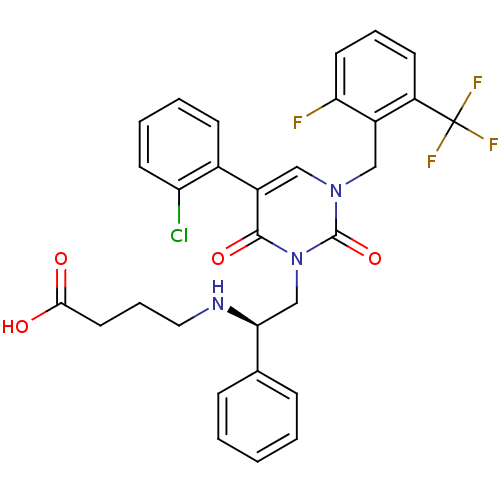
BDBM50260762 (R)-4-(2-(3-(2-fluoro-6-(trifluoromethyl)benzyl)-5-(2-chlorophenyl)-2,6-dioxo-2,3-dihydropyrimidin-1(6H)-yl)-1-phenylethylamino)butanoic acid::CHEMBL450471
SMILES OC(=O)CCCN[C@@H](Cn1c(=O)c(cn(Cc2c(F)cccc2C(F)(F)F)c1=O)-c1ccccc1Cl)c1ccccc1
InChI Key InChIKey=WXJLFSUTNCMCGA-SANMLTNESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50260762
Found 3 hits for monomerid = 50260762
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:Binding affinity to human GnRHRMore data for this Ligand-Target Pair
TargetGonadotropin-releasing hormone receptor(Homo sapiens (Human))
Neurocrine Biosciences
Curated by ChEMBL
Neurocrine Biosciences
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Antagonist activity at human GnRHR expressed in RBL cells assessed as inhibition of GnRH-stimulated calcium fluxMore data for this Ligand-Target Pair
Affinity DataIC50: 1.58E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair