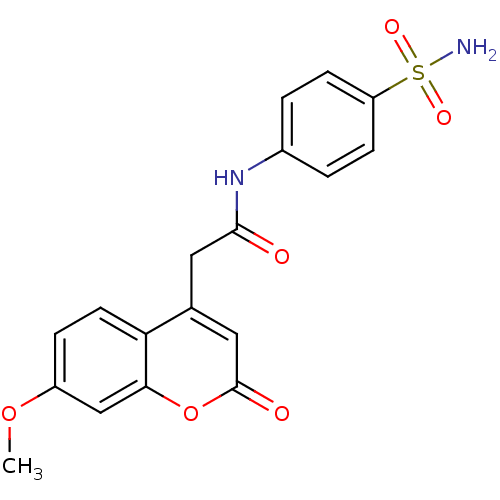
BDBM50263373 2-(7-Methoxy-2-oxo-2H-chromen-4-yl)-N-(4-sulfamoyl-phenyl)-acetamide::2-(7-methoxy-2-oxo-2H-chromen-4-yl)-N-(4-sulfamoylphenyl)acetamide::CHEMBL477822
SMILES COc1ccc2c(c1)OC(=O)C=C2CC(=O)Nc3ccc(cc3)S(=O)(=O)N
InChI Key InChIKey=VZBSCWDKCMOJCR-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50263373
Found 4 hits for monomerid = 50263373
Affinity DataKi: 9nMAssay Description:Inhibition of human recombinant cytosolic carbonic anhydrase 2 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 63nMAssay Description:Inhibition of human recombinant carbonic anhydrase 12 catalytic domain preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 73nMAssay Description:Inhibition of human recombinant cytosolic carbonic anhydrase 1 preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair
Affinity DataKi: 87nMAssay Description:Inhibition of human recombinant carbonic anhydrase 9 catalytic domain preincubated for 15 mins by CO2 hydration methodMore data for this Ligand-Target Pair