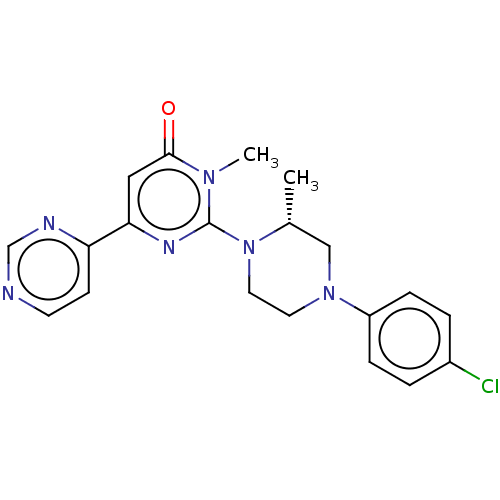
BDBM50269439 CHEMBL4077048
SMILES C[C@@H]1CN(CCN1c1nc(cc(=O)n1C)-c1ccncn1)c1ccc(Cl)cc1
InChI Key InChIKey=VJJBCEKGAXKNSB-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50269439
Found 4 hits for monomerid = 50269439
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human GSK-3beta using prephosphorylated-GS1 peptide as substrate after 1 hr in presence of [gamma-32P]ATP by liquid scintillation spect...More data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of recombinant human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of recombinant human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of recombinant human CYP3A4More data for this Ligand-Target Pair