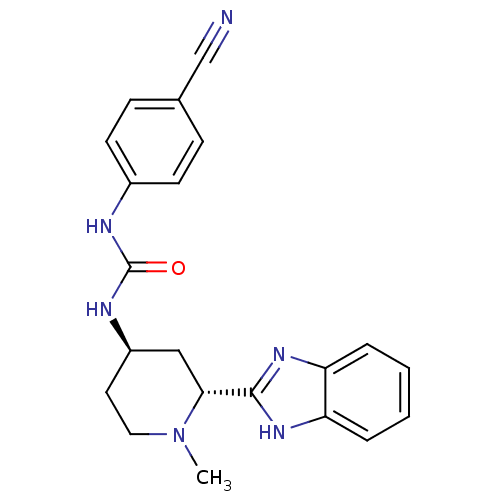
BDBM50385635 CHEMBL2043437
SMILES CN1CC[C@H](C[C@@H]1c1nc2ccccc2[nH]1)NC(=O)Nc1ccc(cc1)C#N
InChI Key InChIKey=SFNSLLSYNZWZQG-UHFFFAOYSA-N
Data 7 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50385635
Found 7 hits for monomerid = 50385635
Affinity DataIC50: 5nMAssay Description:Inhibition of Smo in mouse C3H10T1/2 cells using human recombinant SHH assessed as effect on SMO/SHH transient transcriptional activation after 20 hr...More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C8More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair