BDBM50434789 CHEMBL2386633
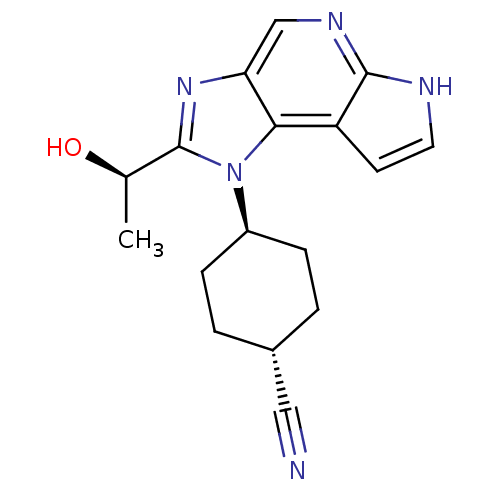
SMILES C[C@H](c1nc2cnc3c(c2n1C4CCC(CC4)C#N)cc[nH]3)O
InChI Key InChIKey=ANDWOIMHOOWCLK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50434789
Found 8 hits for monomerid = 50434789
Affinity DataKi: 1.90nMAssay Description:Inhibition of recombinant JAK1 (unknown origin) using Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr as substrate after 30 mins in presence of ATPMore data for this Ligand-Target Pair
Affinity DataEC50: 120nMAssay Description:Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation incubated for 20 mins followed by IL6 challenge for...More data for this Ligand-Target Pair
Affinity DataEC50: 130nMAssay Description:Inhibition of JAK1 in human TF1 cells assessed as inhibition of IL6-induced STAT3 phosphorylation incubated for 20 mins followed by IL6 challenge for...More data for this Ligand-Target Pair
Affinity DataKi: 280nMAssay Description:Inhibition of JAK3 (unknown origin) using Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg as substrate after 30 mins in presence of ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Reversible inhibition of CYP2C9 in human liver microsomes using warfarin as substrate by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Reversible inhibition of CYP2C19 in human liver microsomes using mephenytoin as substrate by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Reversible inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate by LC/MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Reversible inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate by LC/MS/MS analysisMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)