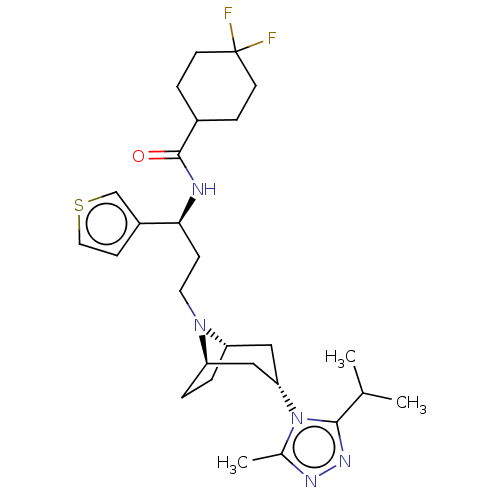
BDBM50464136 CHEMBL4249798
SMILES Cc1nnc(n1C2C[C@H]3CC[C@@H](C2)N3CC[C@@H](c4ccsc4)NC(=O)C5CCC(CC5)(F)F)C(C)C
InChI Key InChIKey=KESMRFWRGXZDEF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50464136
Found 8 hits for monomerid = 50464136
TargetC-C chemokine receptor type 5(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Antagonist activity at CCR5 (unknown origin) expressed in HEK293 cells co-expressing Galpha16 assessed as inhibition of RANTES-induced calcium flux p...More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using testosterone as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin) using midazolam as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human ERG expressed in CHO cells at holding potential of -80 mV after 60 secs by patch clamp assayMore data for this Ligand-Target Pair