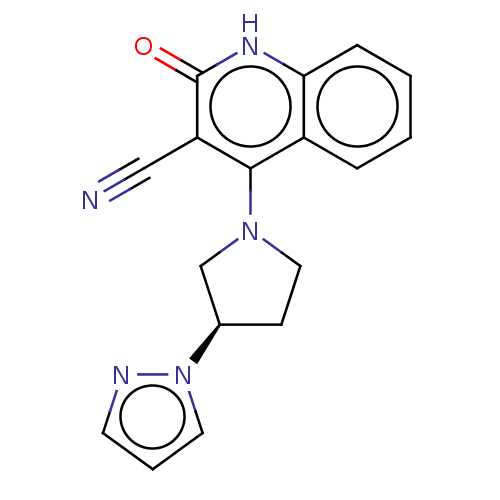
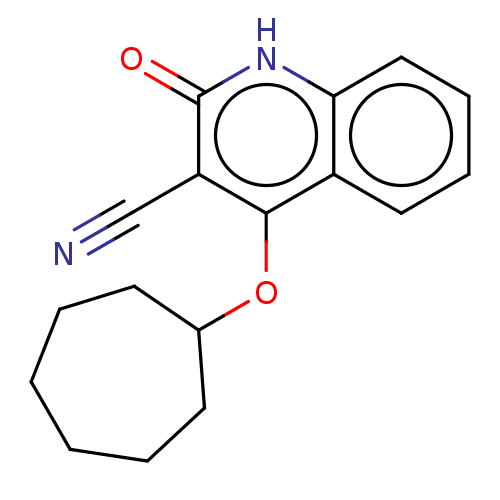
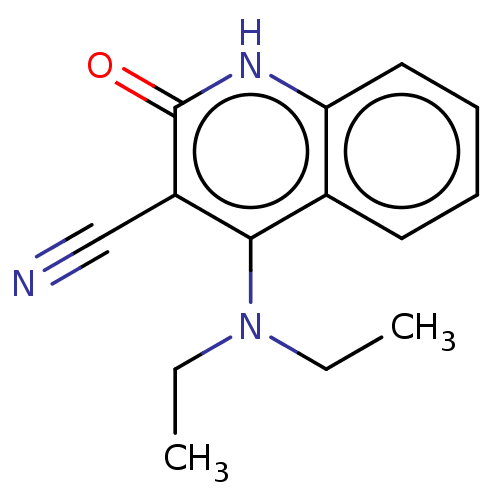
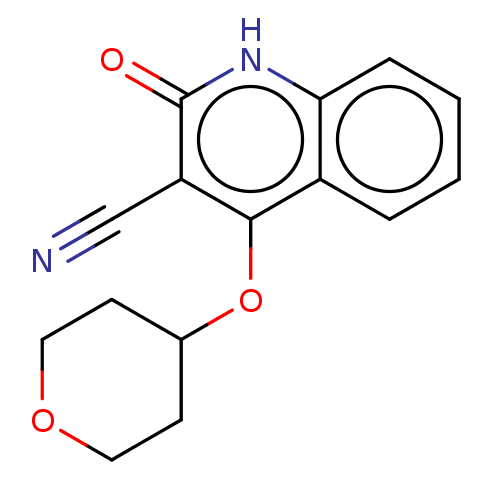
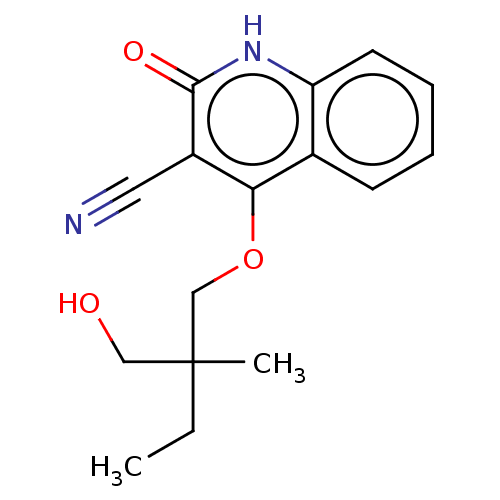
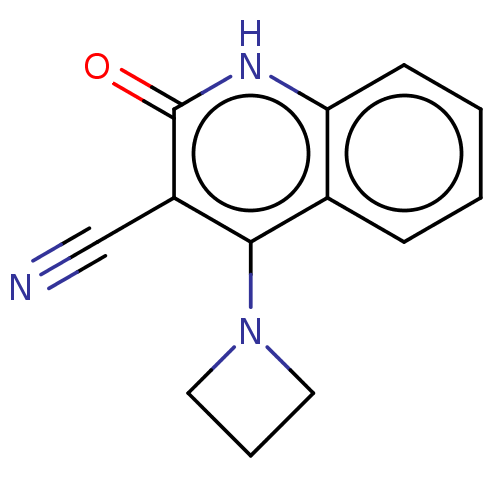
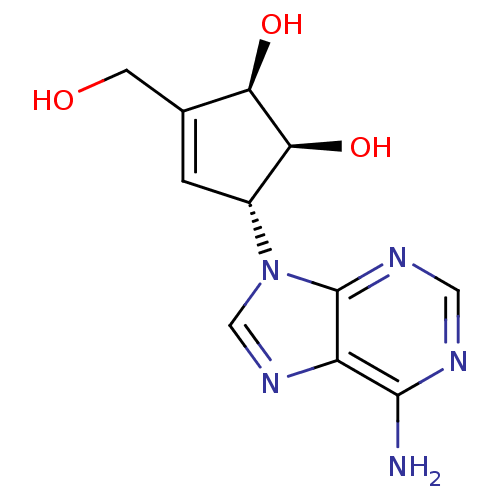
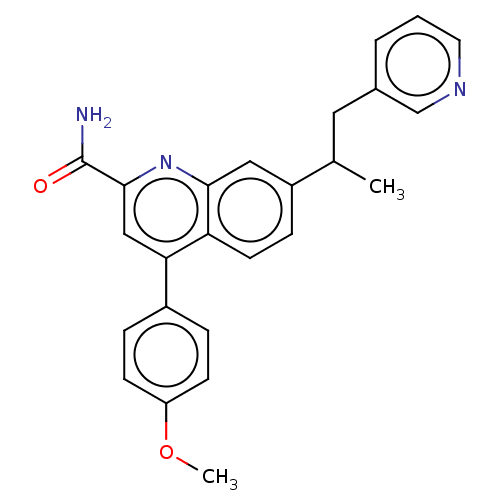
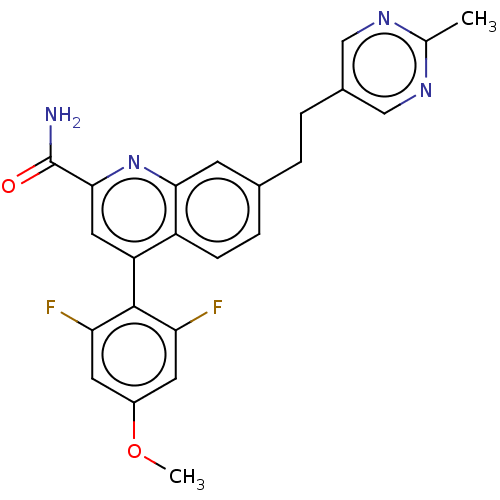
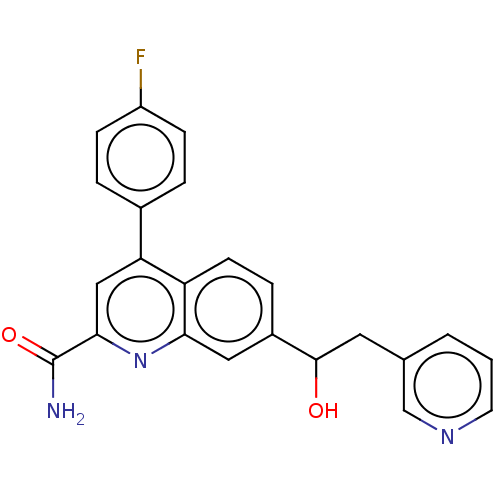
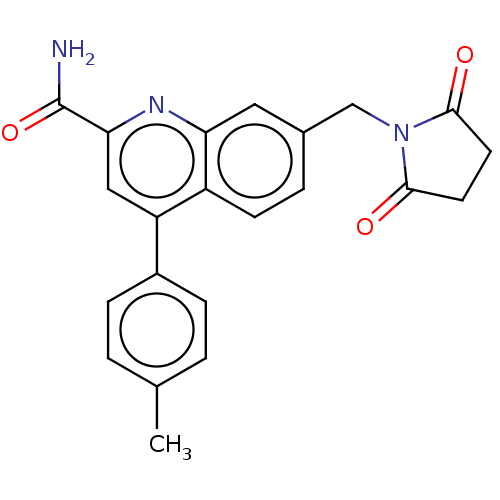
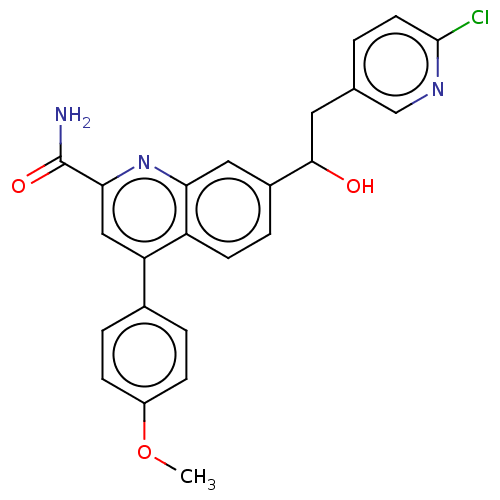
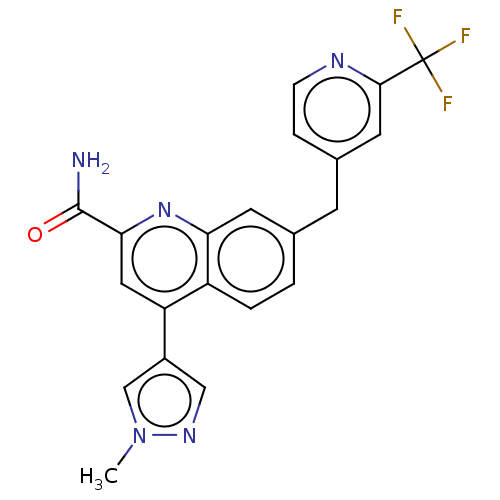
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 3.40nMAssay Description:The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny...More data for this Ligand-Target Pair
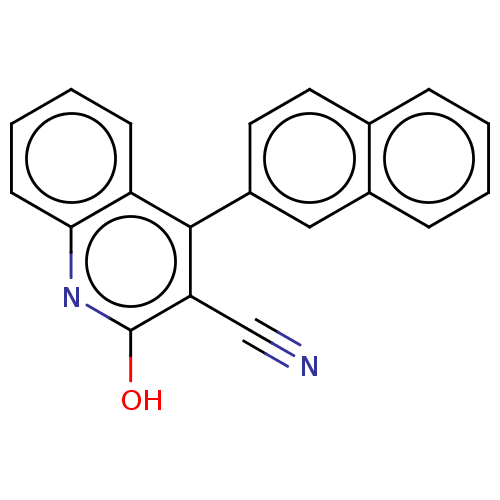
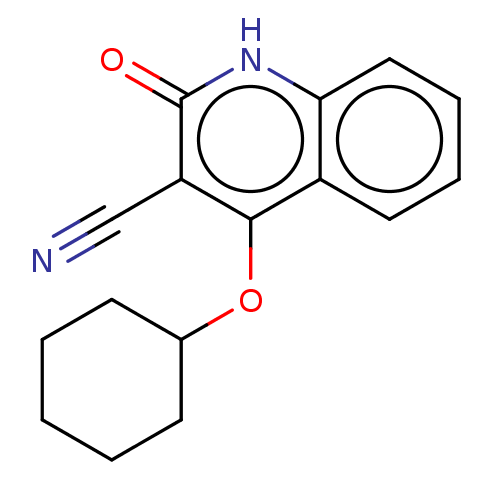
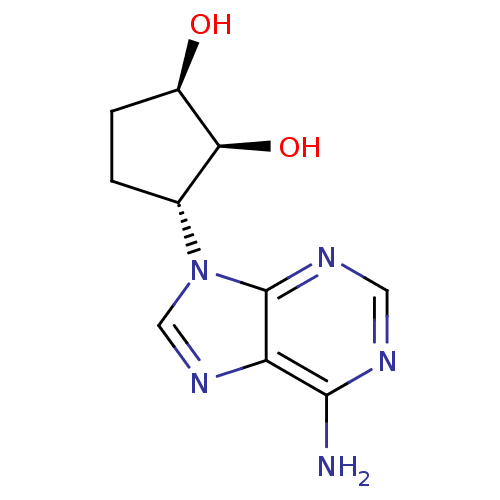
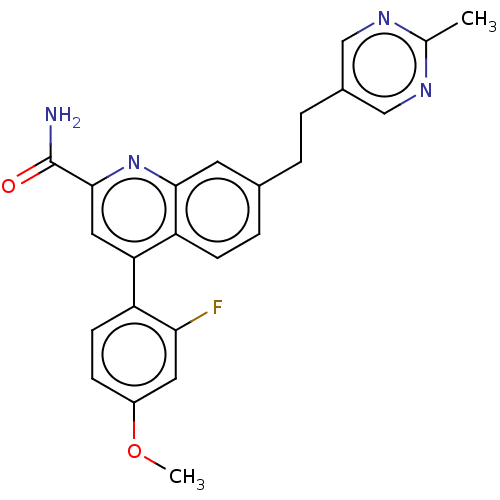
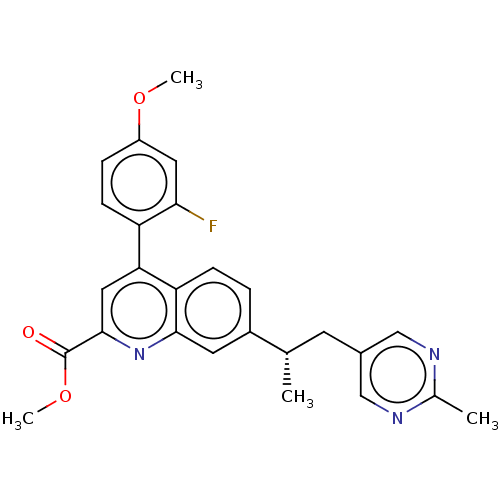
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 7.20nMAssay Description:The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny...More data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Rhesus PDE9A2 was amplified from rhesus whole brain cDNA (Biochain Institute) essentially as described in Hutson, et al. Neuropharmacology (2011) 61(...More data for this Ligand-Target Pair
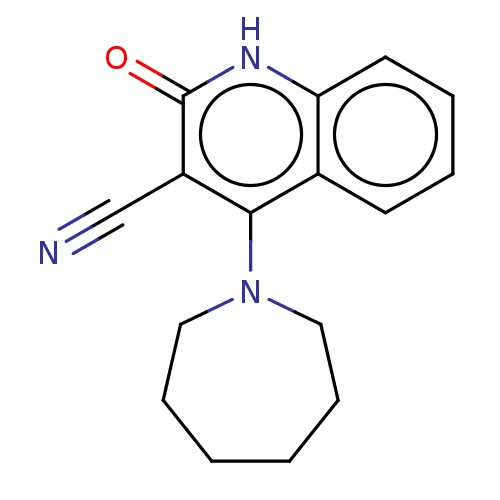
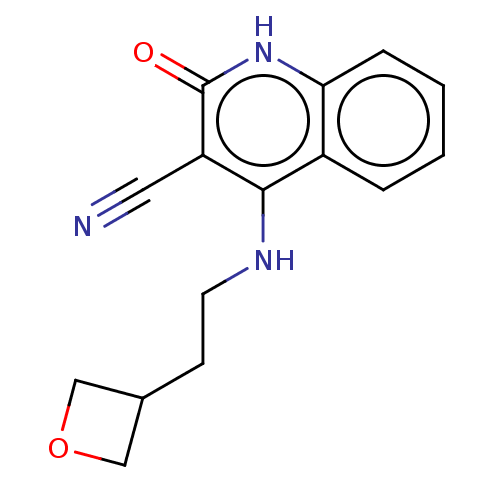
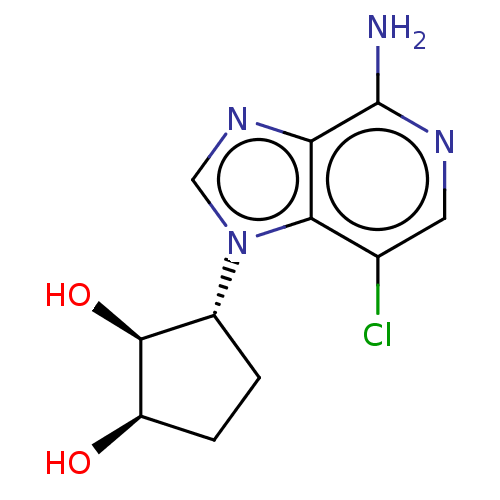
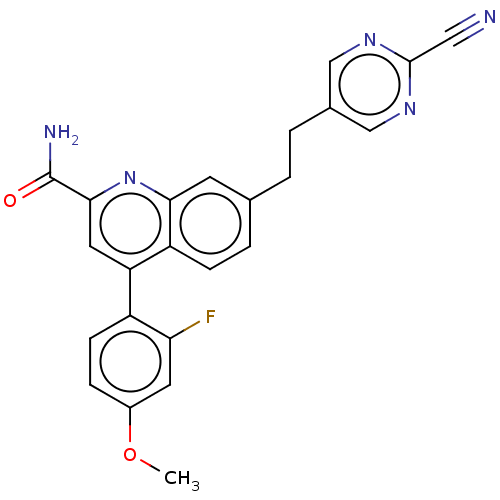
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 8.90nMAssay Description:The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny...More data for this Ligand-Target Pair
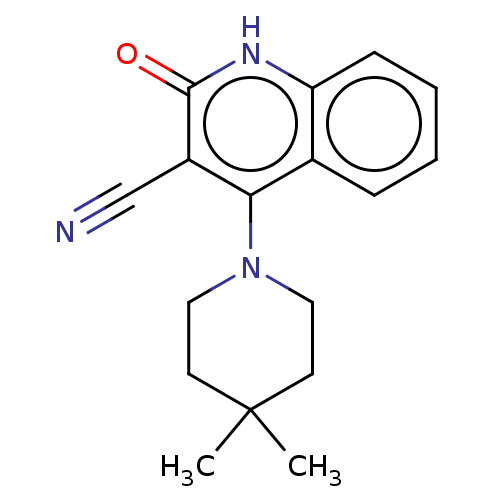
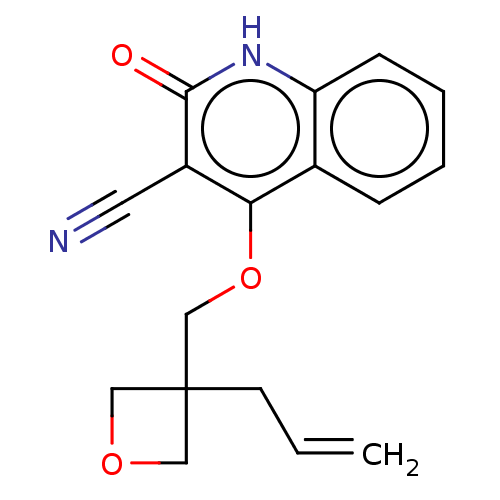
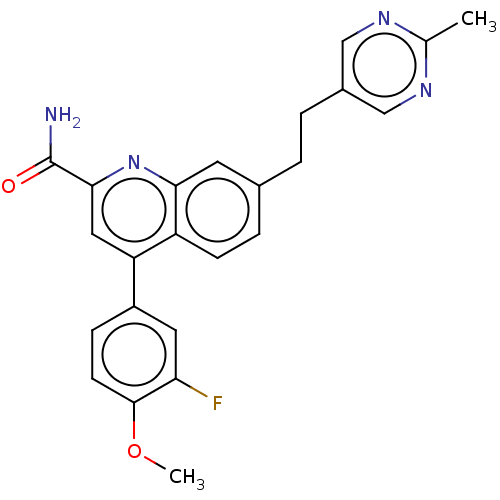
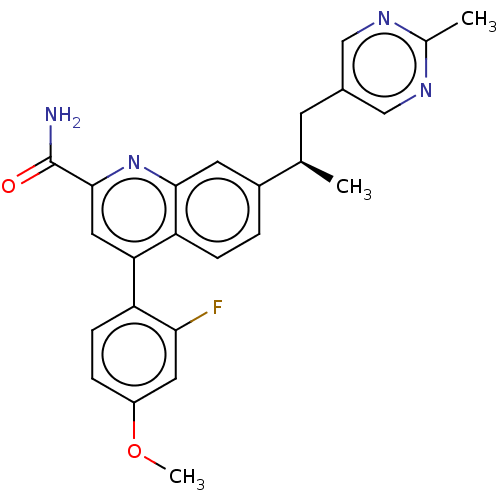
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 14.8nMAssay Description:The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny...More data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Rhesus PDE9A2 was amplified from rhesus whole brain cDNA (Biochain Institute) essentially as described in Hutson, et al. Neuropharmacology (2011) 61(...More data for this Ligand-Target Pair
Affinity DataKi: 26.3nMAssay Description:Rhesus PDE9A2 was amplified from rhesus whole brain cDNA (Biochain Institute) essentially as described in Hutson, et al. Neuropharmacology (2011) 61(...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 29nMAssay Description:Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 50.3nMAssay Description:Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 66nMAssay Description:The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny...More data for this Ligand-Target Pair
Affinity DataKi: 71.1nMAssay Description:Rhesus PDE9A2 was amplified from rhesus whole brain cDNA (Biochain Institute) essentially as described in Hutson, et al. Neuropharmacology (2011) 61(...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 87.8nMAssay Description:Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol...More data for this Ligand-Target Pair
Affinity DataKi: 88.9nMAssay Description:Rhesus PDE9A2 was amplified from rhesus whole brain cDNA (Biochain Institute) essentially as described in Hutson, et al. Neuropharmacology (2011) 61(...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 135nMAssay Description:Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 182nMAssay Description:Human PDE9 (PDE9A2, GenBank Accession No. NM_001001567), full length with N-terminal GST tag, was purchased from BPS Bioscience. The fluorescence pol...More data for this Ligand-Target Pair
TargetIsoform PDE9A2 of High affinity cGMP-specific 3',5'-cyclic phosphodiesterase 9A (PDE9A2)(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataKi: 444nMAssay Description:The fluorescence polarization assay for cyclic nucleotide phosphodiesterases was performed using an IMAP® FP kit supplied by Molecular Devices, Sunny...More data for this Ligand-Target Pair
Affinity DataKi: 5.55E+3nMAssay Description:Rhesus PDE9A2 was amplified from rhesus whole brain cDNA (Biochain Institute) essentially as described in Hutson, et al. Neuropharmacology (2011) 61(...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of recombinant human AHCY using SAH as substrate assessed as formation of homocysteine after 10 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70nMAssay Description:Inhibition of AHCY in human SH-SY5Y cells assessed as formation of homocysteine after 48 hrs by HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 4nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
TargetGuanine nucleotide-binding protein subunit alpha-15 [Y147C]/Metabotropic glutamate receptor 2(Homo sapiens (Human))
Merck Sharp & Dohme
US Patent
Merck Sharp & Dohme
US Patent
Affinity DataIC50: 5nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR...More data for this Ligand-Target Pair