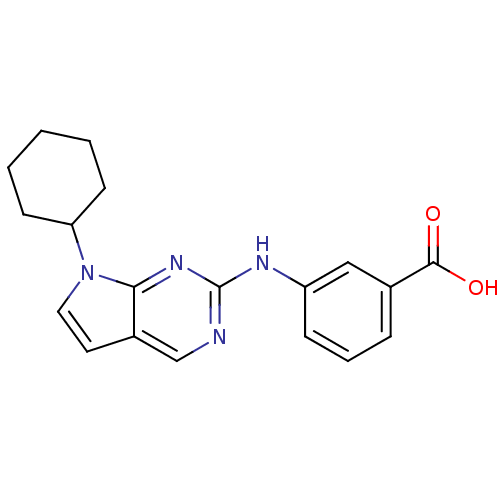
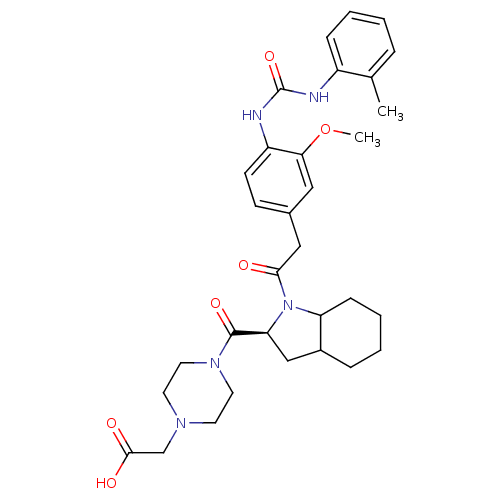
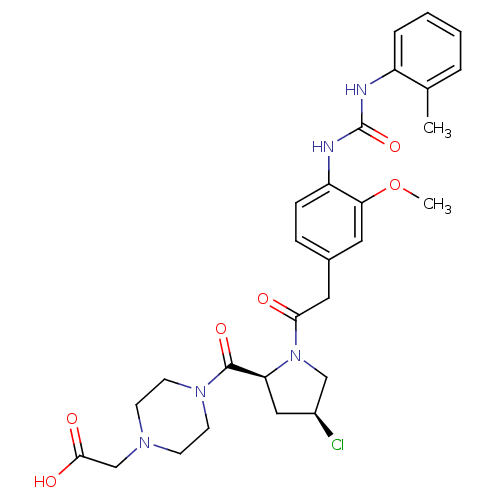
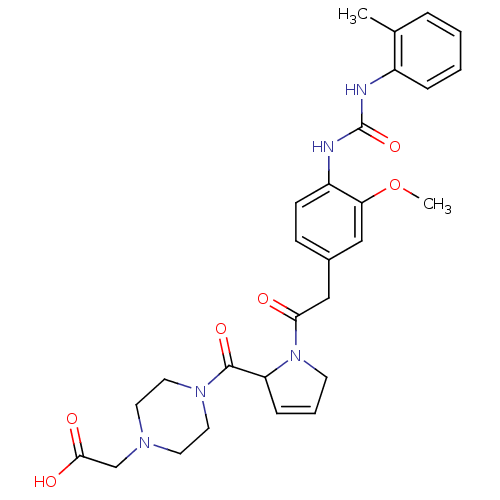
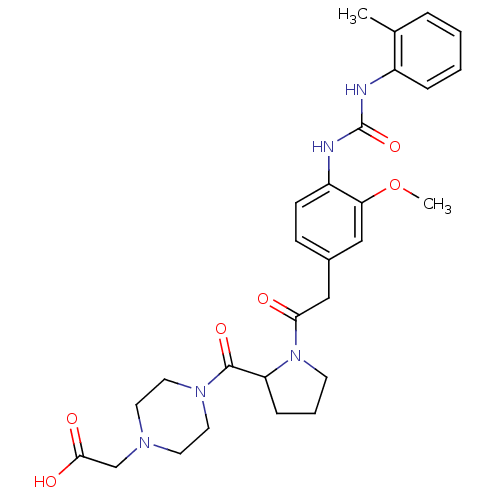
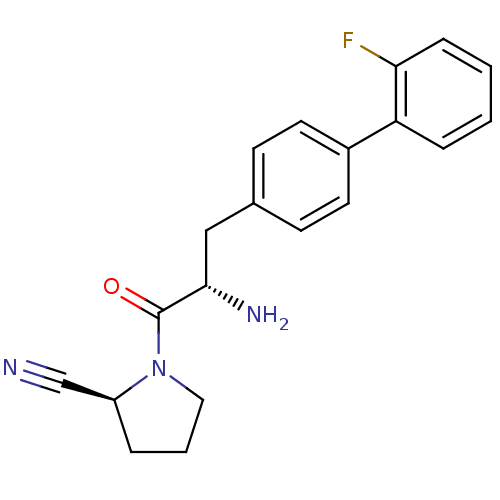
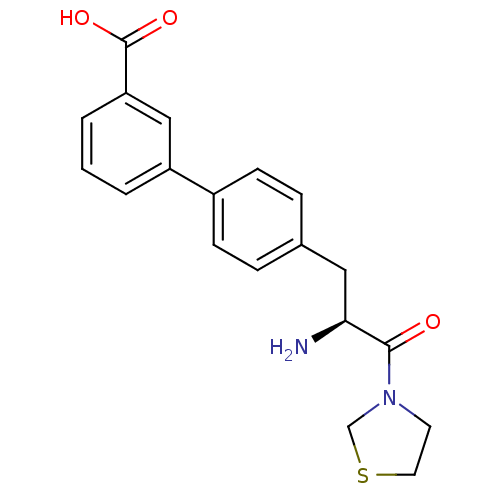
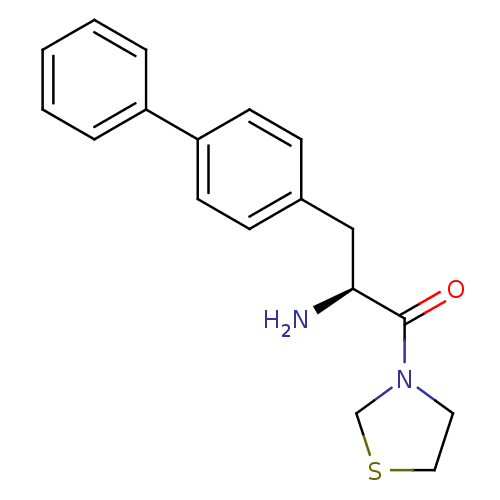
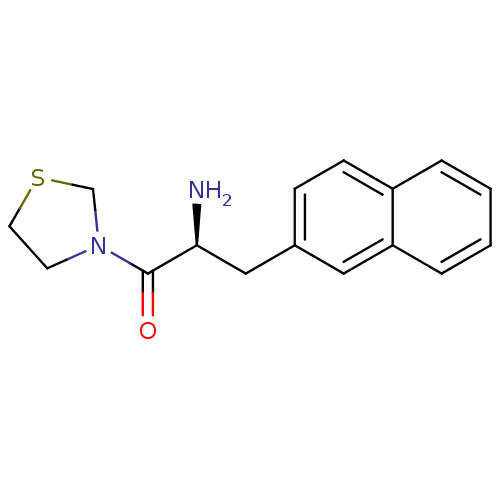
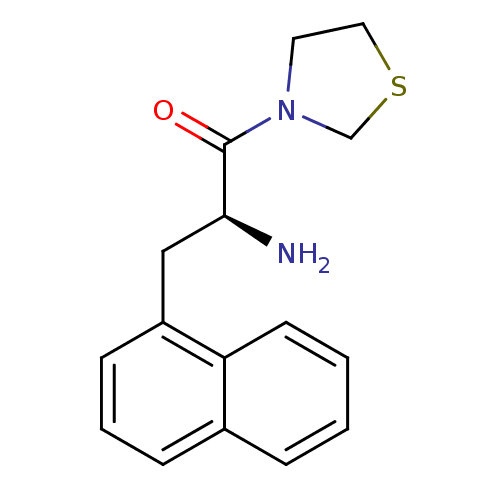
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Antagonistic activity of the compound against LTB4 receptor using guinea pig (GP) spleen cell membraneMore data for this Ligand-Target Pair
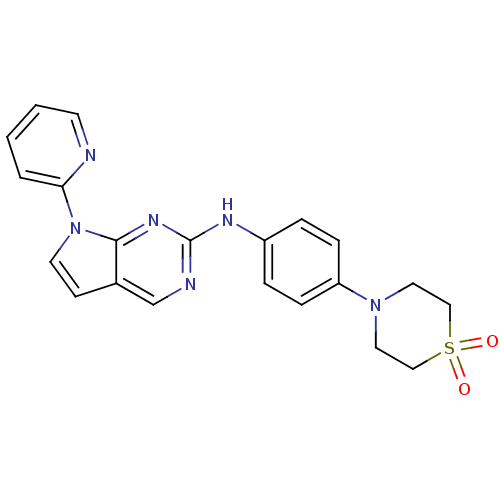
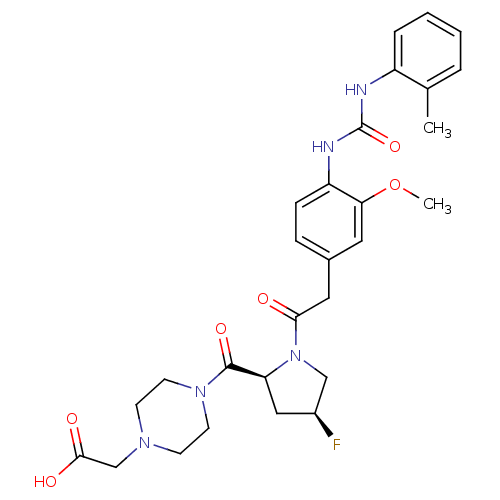
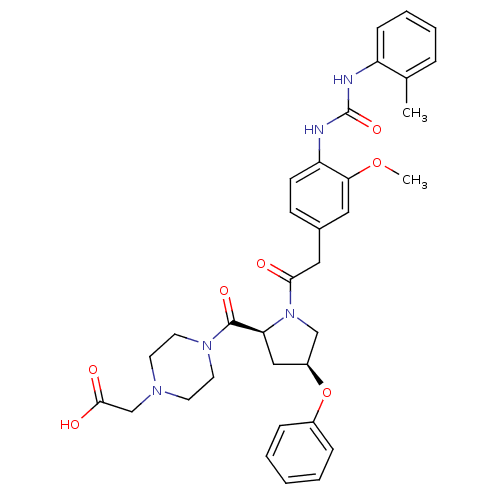
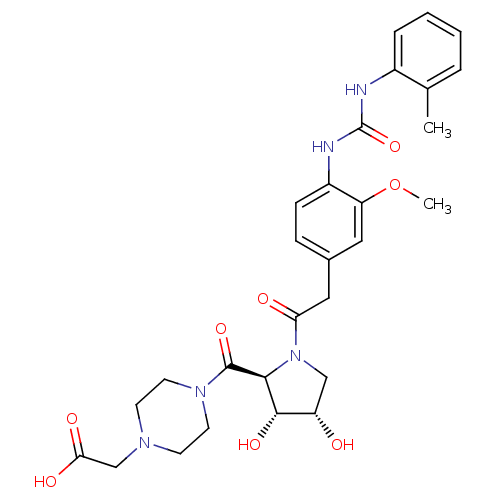
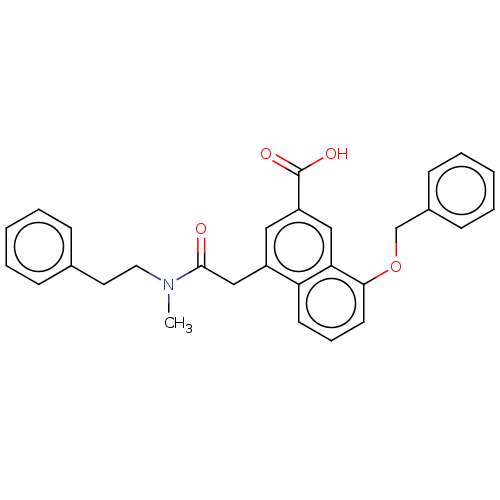
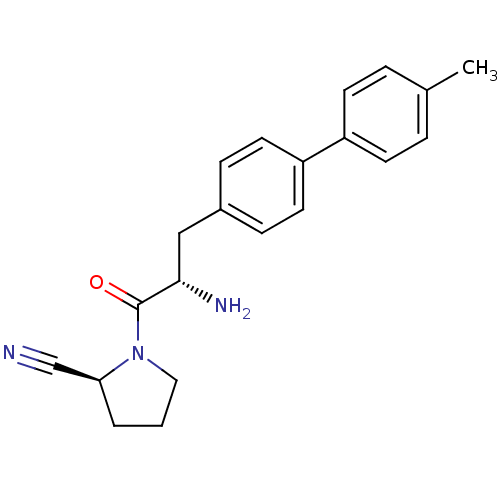
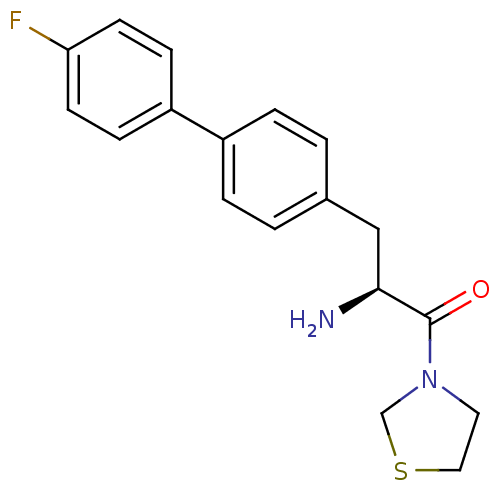
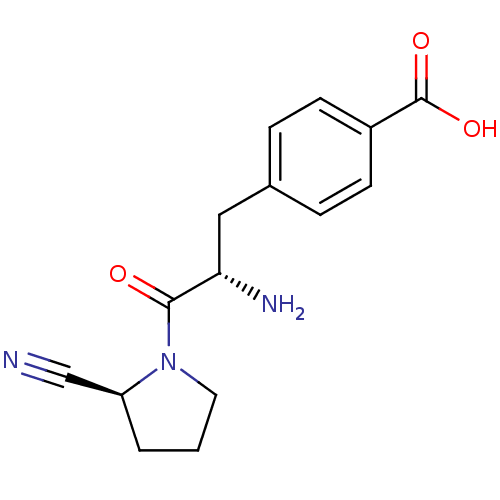
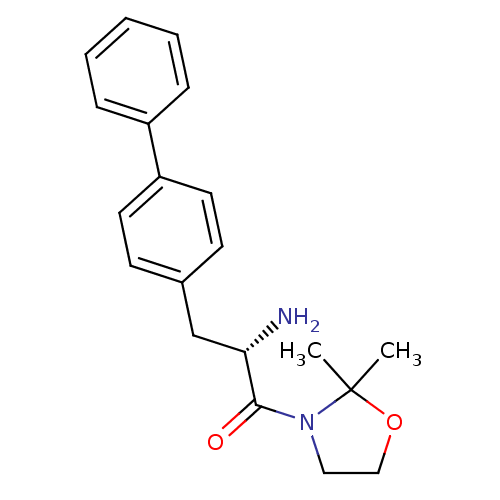
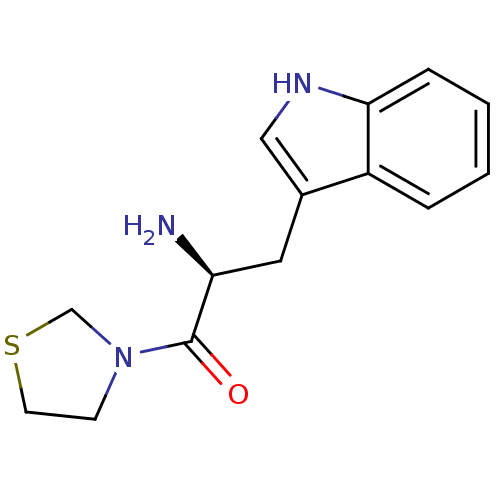
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Antagonistic activity of the compound against monkey neutrophil LTB4 receptor 2 min after an iv dose of 3 mg/kg .More data for this Ligand-Target Pair
Affinity DataKi: 2.20nM ΔG°: -49.4kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 3.10nM ΔG°: -48.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 5.30nM ΔG°: -47.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 13nM ΔG°: -45.0kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -43.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 26nM ΔG°: -43.3kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 27nM ΔG°: -43.2kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 34nM ΔG°: -42.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 36nM ΔG°: -42.5kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 63nM ΔG°: -41.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 96nM ΔG°: -40.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 160nM ΔG°: -38.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 166nM ΔG°: -38.7kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 170nM ΔG°: -38.6kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 310nM ΔG°: -37.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 355nM ΔG°: -36.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 360nM ΔG°: -36.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 470nM ΔG°: -36.1kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 980nM ΔG°: -34.3kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 1.16E+3nM ΔG°: -33.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: 8.90E+3nM ΔG°: -28.8kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
Affinity DataKi: >1.28E+4nM ΔG°: >-27.9kJ/molepH: 7.4 T: 2°CAssay Description:The progress of DPPIV inhibition by compounds was measured by recording the liberation of free pNA at 405 nm. IC50 was determined from the slope of r...More data for this Ligand-Target Pair
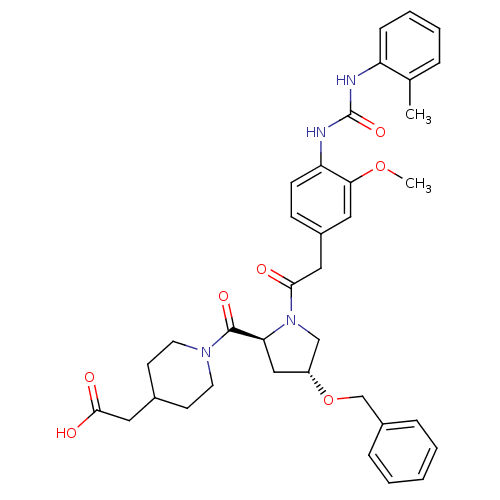
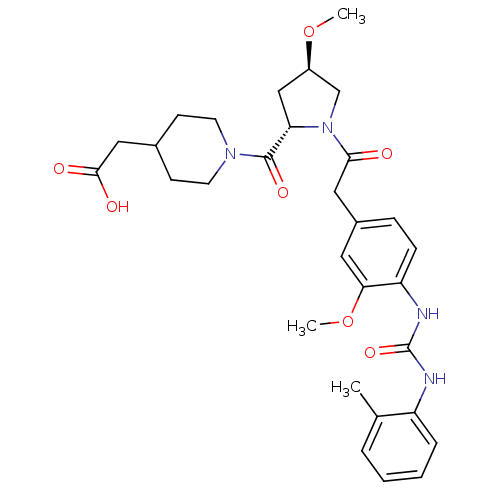
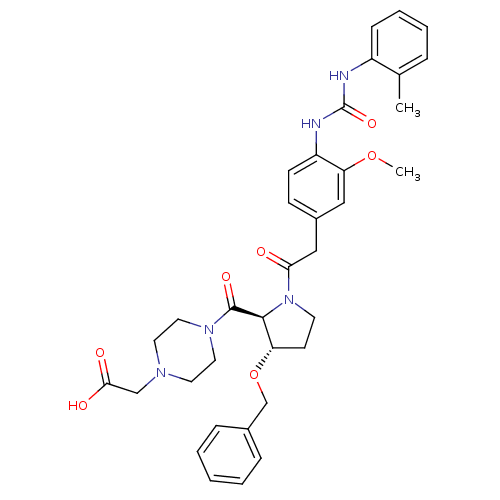
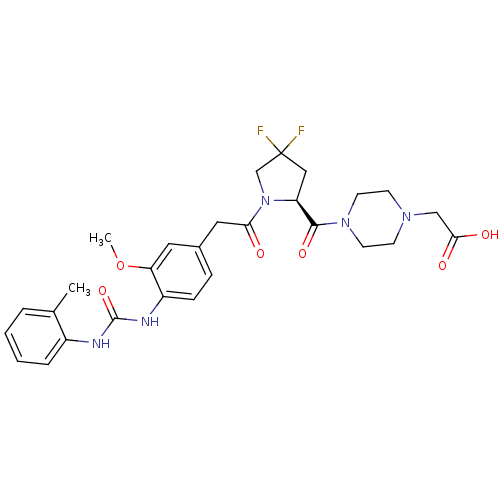
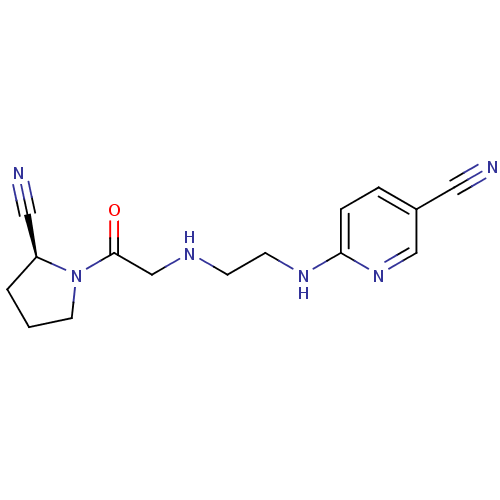
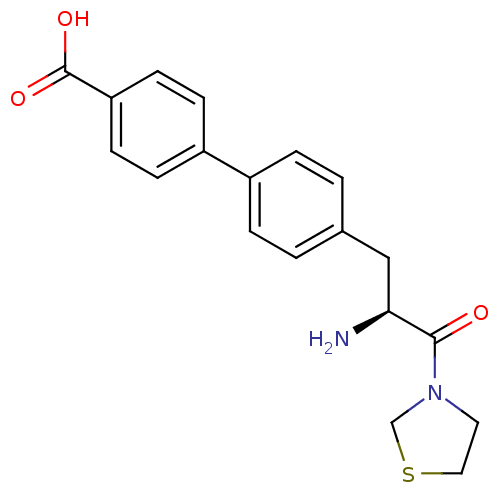
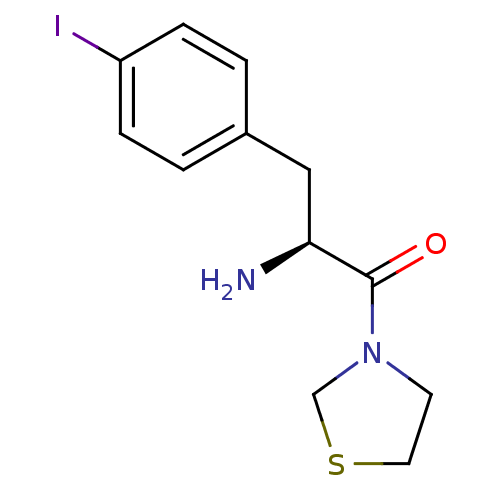
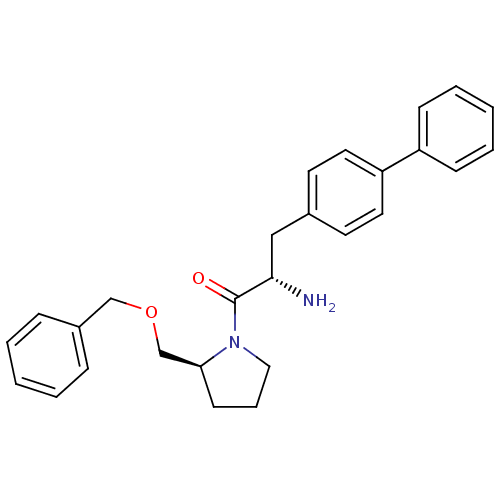
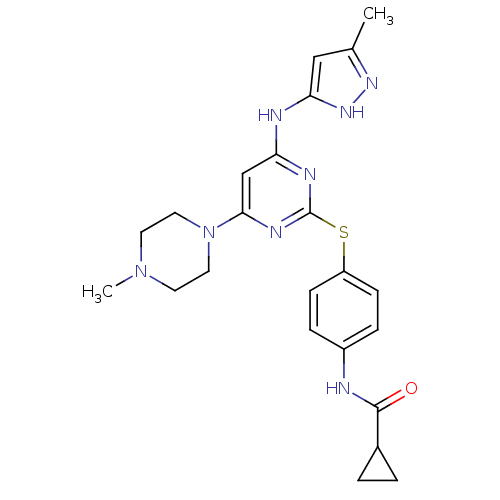
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Affinity DataIC50: 0.360nMAssay Description:Affinity of the compound for human PMN LTB-4 receptors.More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMpH: 7.5 T: 2°CAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of serine 10 of histone-H3 by purified recombinant murine Aurora-A enzyme....More data for this Ligand-Target Pair
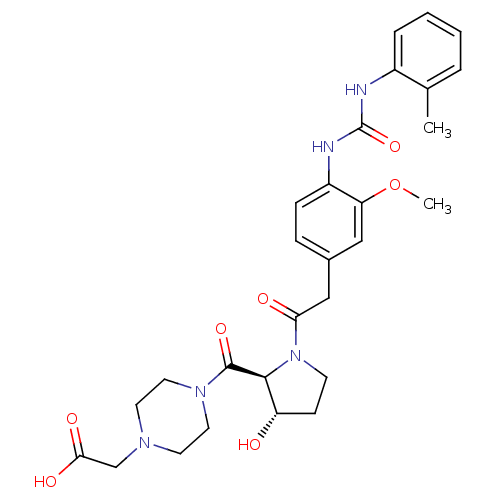
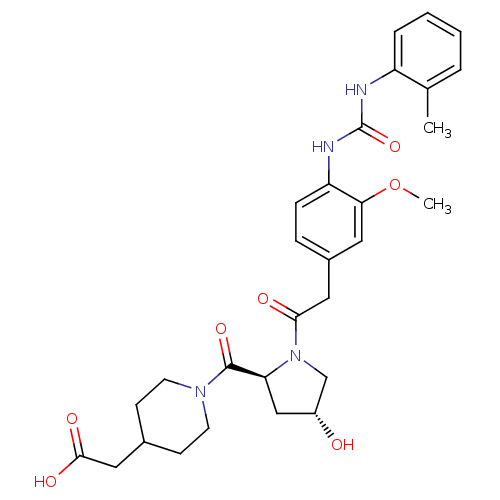
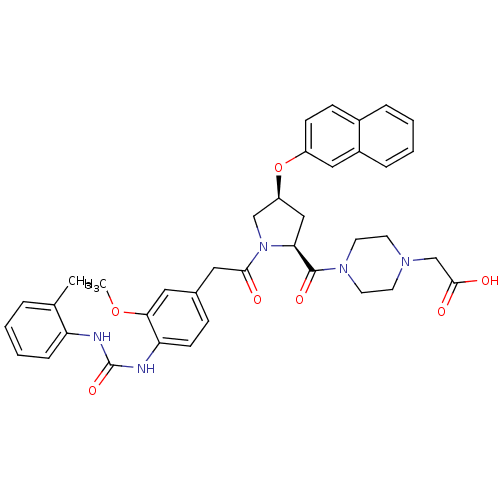
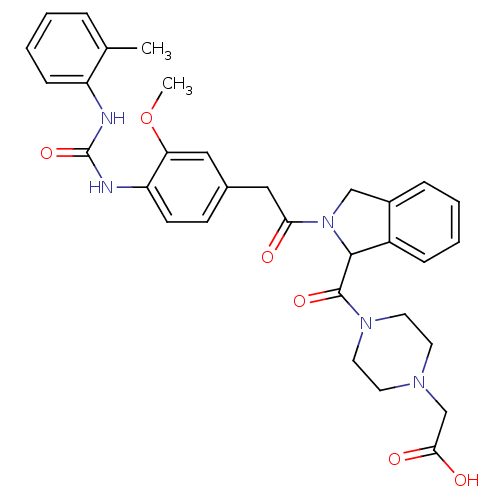
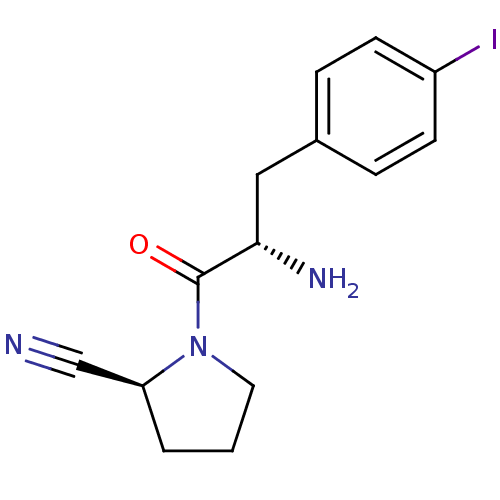
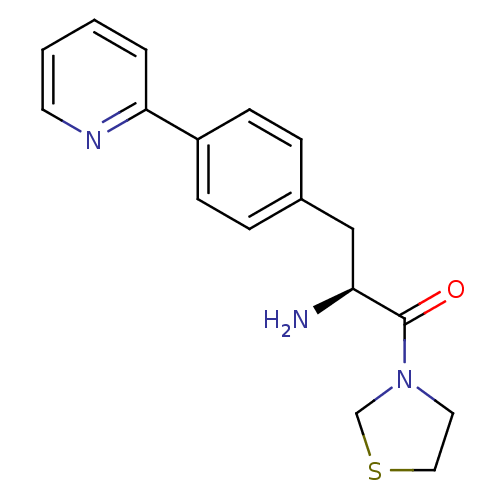
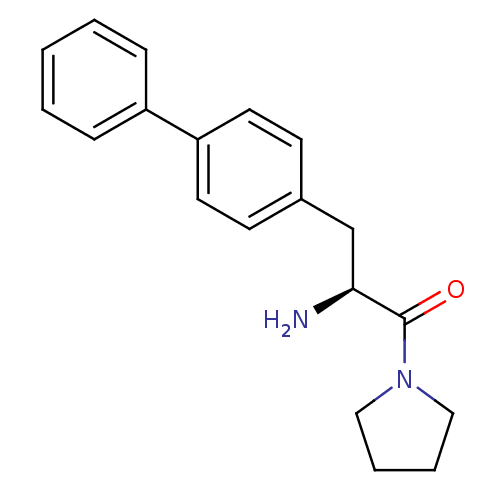
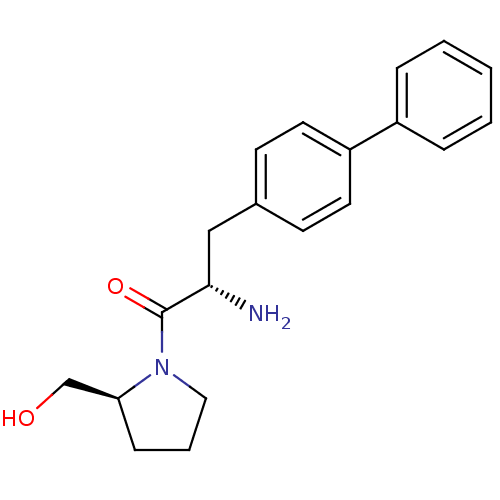
TargetLeukotriene B4 receptor 1(Homo sapiens (Human))
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Rhone-Poulenc Rorer Central Research
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Concentration of the compound inhibiting 1 nM LTB4-induced aggregation in GP polymorphonuclear (PMN) leukocytes.More data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 0.960nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: <1nMAssay Description:The compounds were tested for their ability to inhibit the phosphorylation of poly(Glu:Tyr) by purified recombinant human FLT3. The extent of phospho...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Inhibition of VLA-4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMpH: 7.5 T: 2°CAssay Description:The 4B4 cells expressing VLA-4 were distributed into each well of a 96-well culture plate for 2 days. Compound and Eu-labeled Human VCAM-1/Fc Chimera...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of VLA-4 receptor expressed in CHO cellsMore data for this Ligand-Target Pair

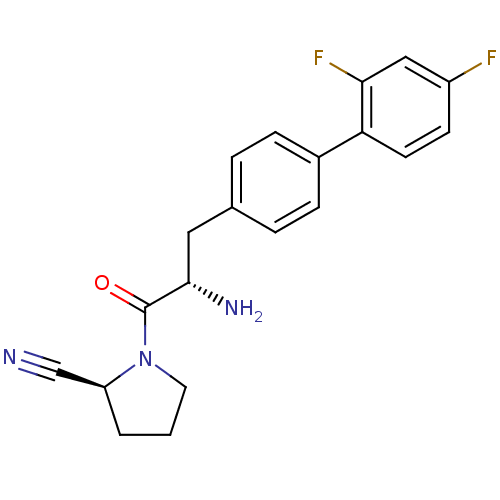
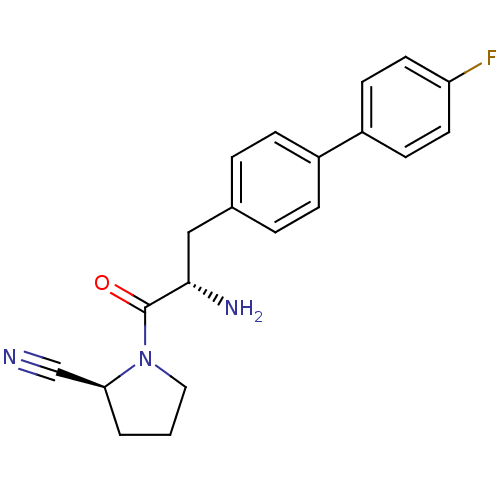


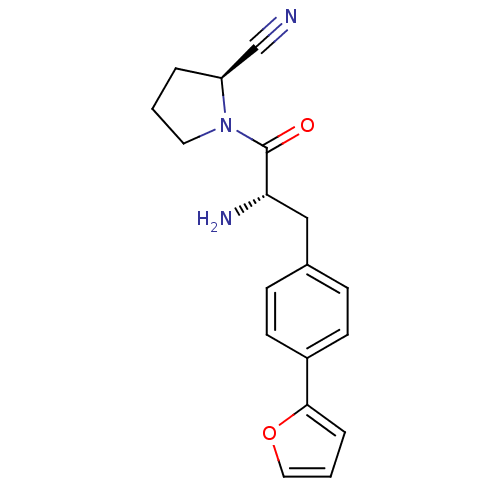
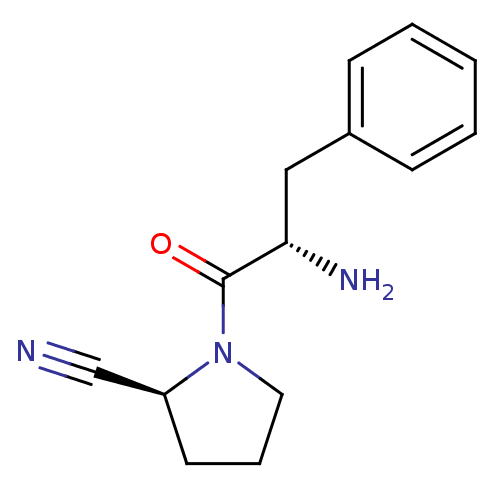
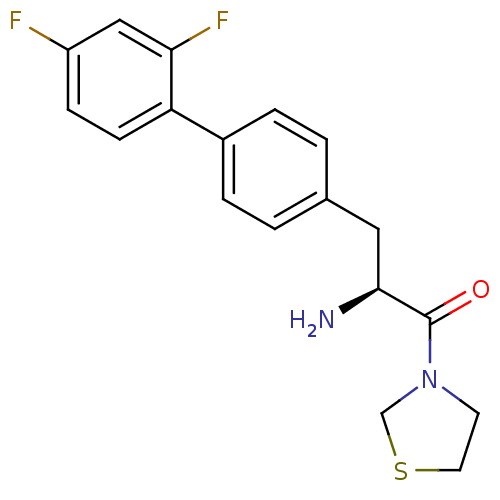
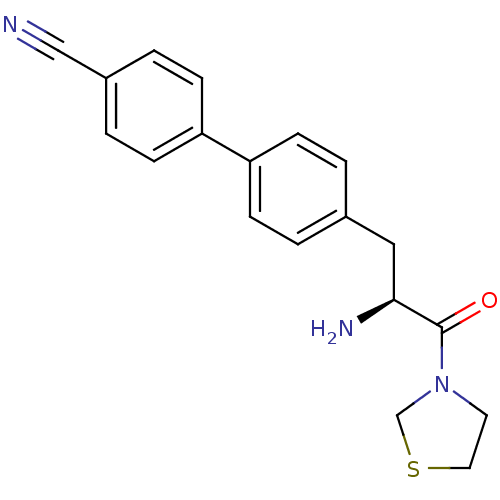

 3D Structure (crystal)
3D Structure (crystal)