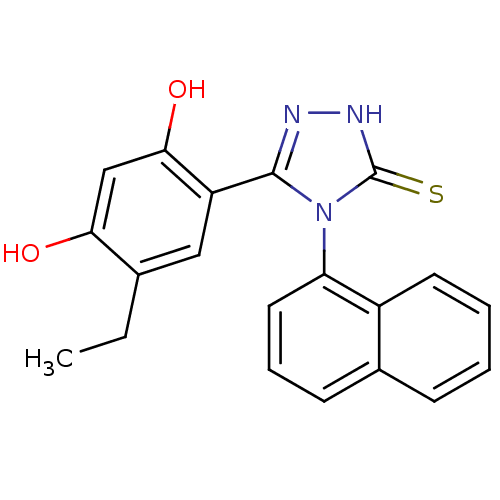
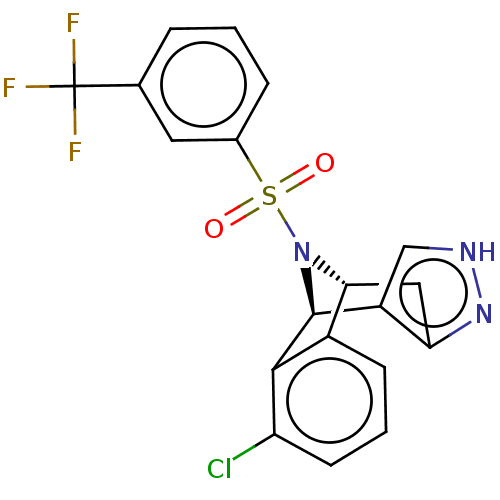
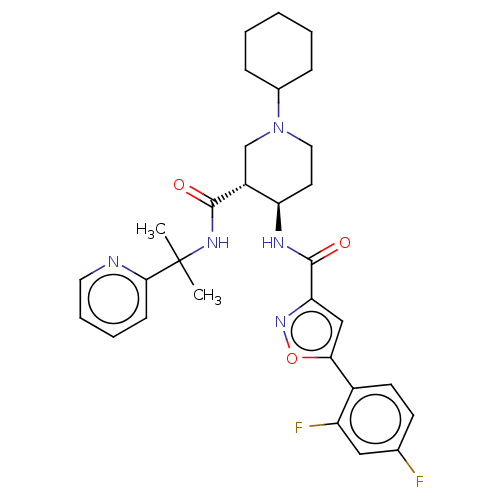
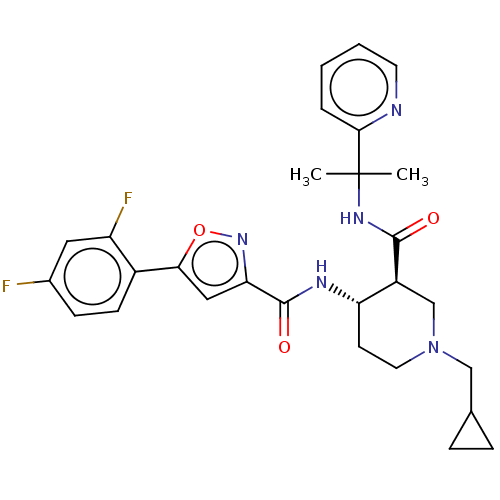
Affinity DataKi: 0.160nMAssay Description:Antagonist activity at recombinant human H3 receptor expressed in CHO-K1 cell membranes incubated for 60 mins by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 43nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Inhibition of androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 93nMAssay Description:Inhibition of androgen receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 95nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 96nMAssay Description:Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine HydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 101nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine HydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 111nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 134nMAssay Description:Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine HydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 224nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 273nMAssay Description:Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 360nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 410nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 570nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 570nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 670nMAssay Description:concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson methodMore data for this Ligand-Target Pair
Affinity DataKi: 681nMAssay Description:Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine HydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 710nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 770nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivationMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine HydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 1.70E+3nMAssay Description:Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivationMore data for this Ligand-Target Pair
Affinity DataKi: 1.93E+3nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 2.60E+3nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nMAssay Description:The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compoundMore data for this Ligand-Target Pair
Affinity DataKi: 3.60E+3nMAssay Description:The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compoundMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+3nMAssay Description:Kinetic constant calculated from the pseudo-first-order rate constant(k app) for S-adenosyl-homocysteine hydrolase inactivationMore data for this Ligand-Target Pair
Affinity DataKi: 3.90E+3nMAssay Description:The kinetic constant for inhibition of S-adenosyl-homocysteine hydrolase by the compoundMore data for this Ligand-Target Pair
Affinity DataKi: 4.50E+3nMAssay Description:Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.98E+4nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 4.34E+4nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataKi: 4.85E+4nMAssay Description:Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant.More data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMpH: 7.4Assay Description:To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMpH: 7.4Assay Description:To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMpH: 7.4Assay Description:To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMpH: 7.4Assay Description:To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)...More data for this Ligand-Target Pair
Affinity DataIC50: 0.220nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Tango CXCR7-bla U2OS cells are detached from culture dishes with 0.05% trypsin-EDTA and collected in growing medium (McCoy's 5A 90% (v/v), dialyz...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of gamma-secretase in human IMR32 cell membrane using APP as substrate after 2 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMpH: 7.4Assay Description:To assess the affinity of compounds binding to Hsp90, we measured their ability to compete with the binding of a fluorescent analog of GA (GM-Bodipy)...More data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Tango CXCR7-bla U2OS cells are detached from culture dishes with 0.05% trypsin-EDTA and collected in growing medium (McCoy's 5A 90% (v/v), dialyz...More data for this Ligand-Target Pair

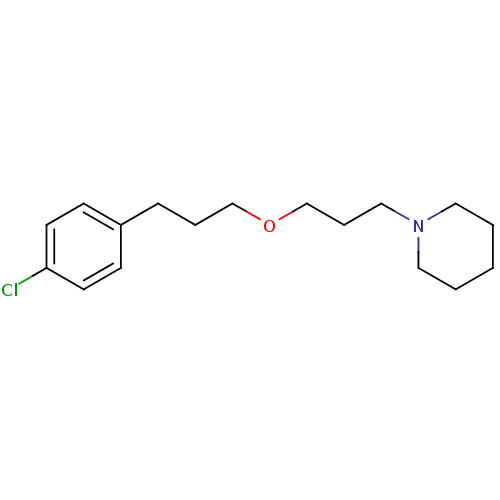
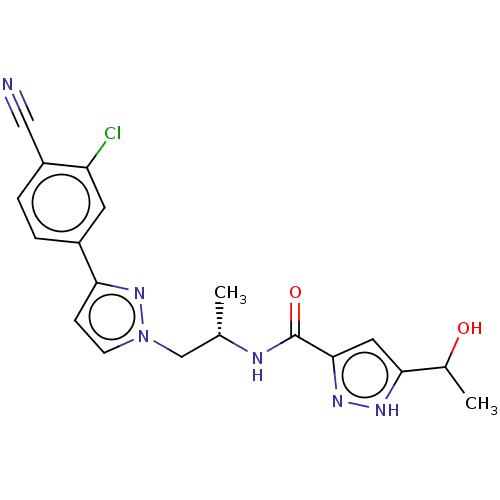
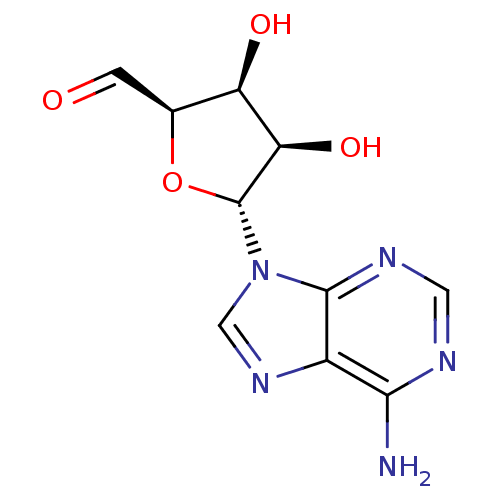

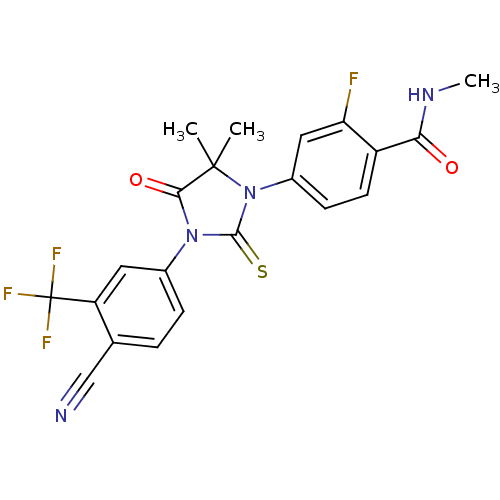

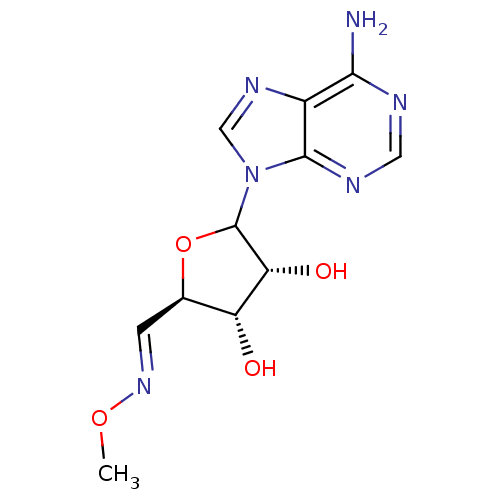
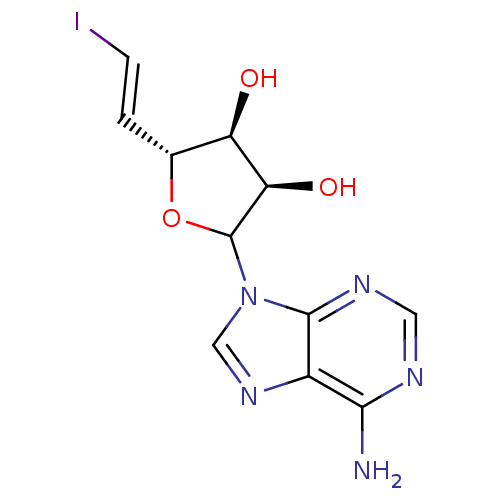
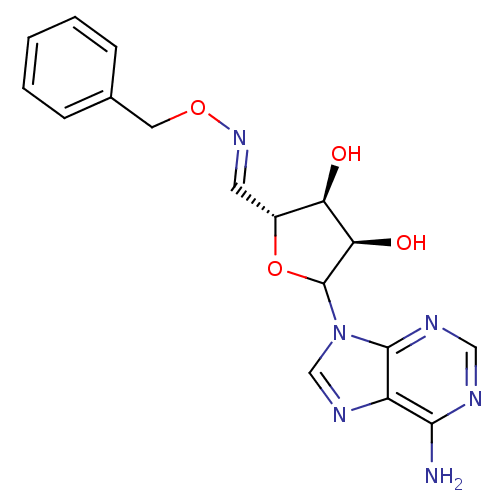
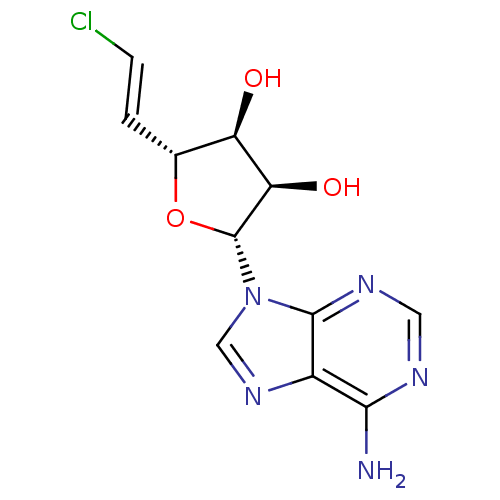

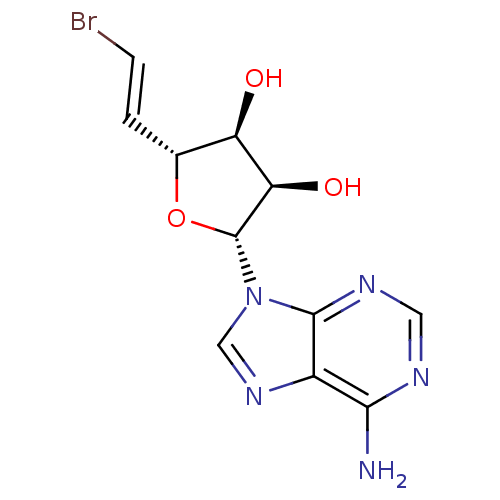

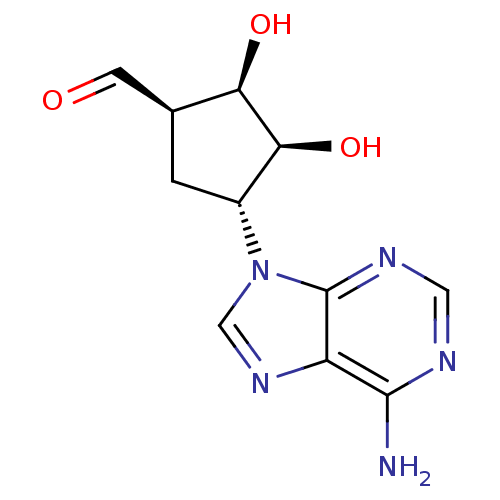
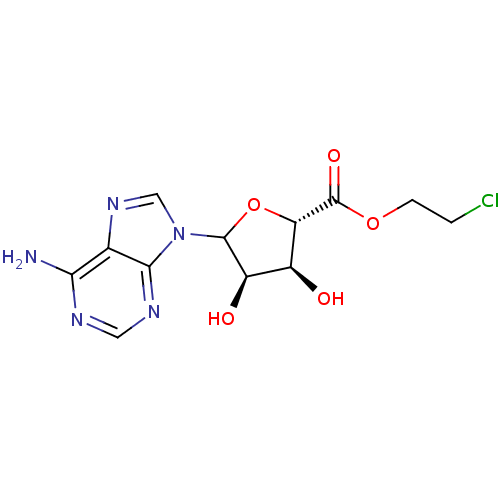
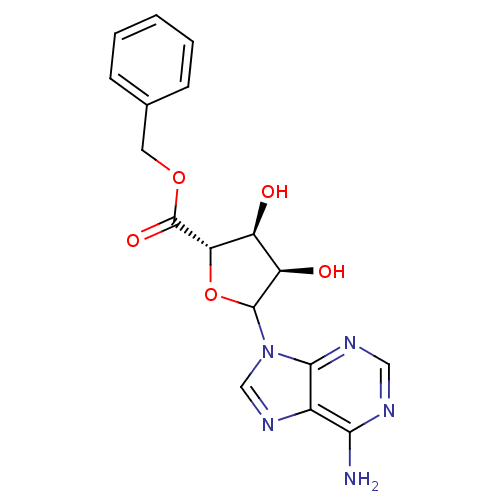

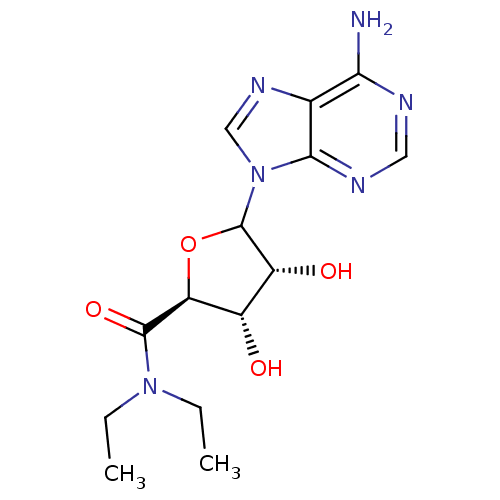
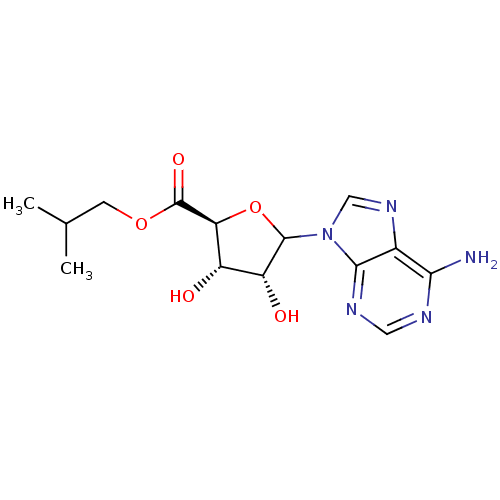
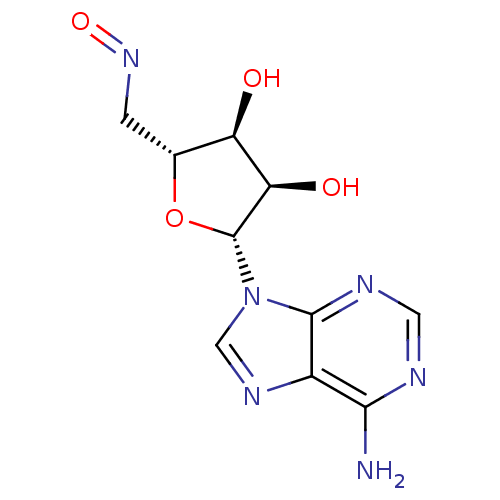
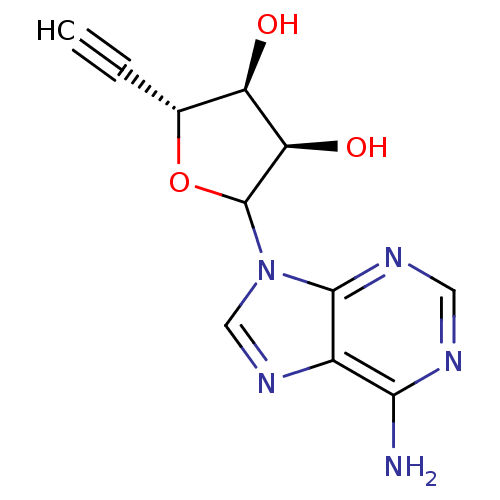
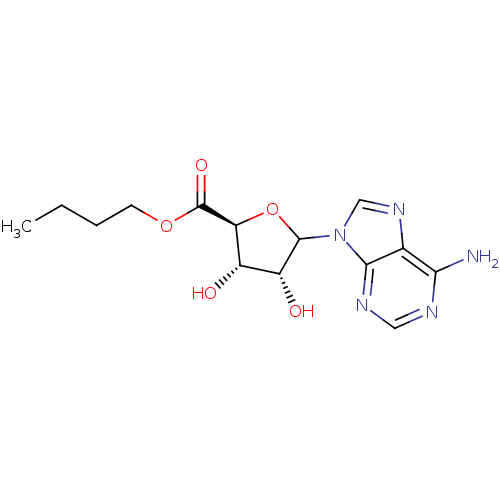
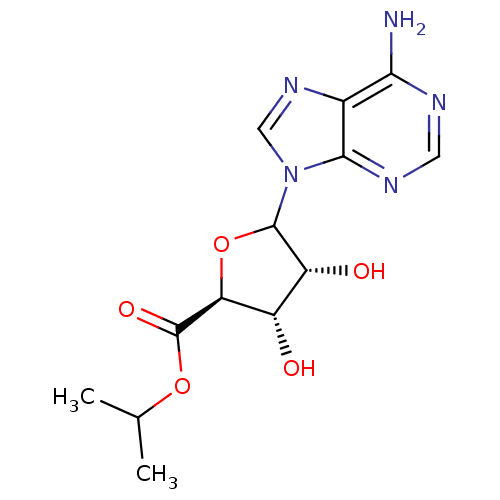
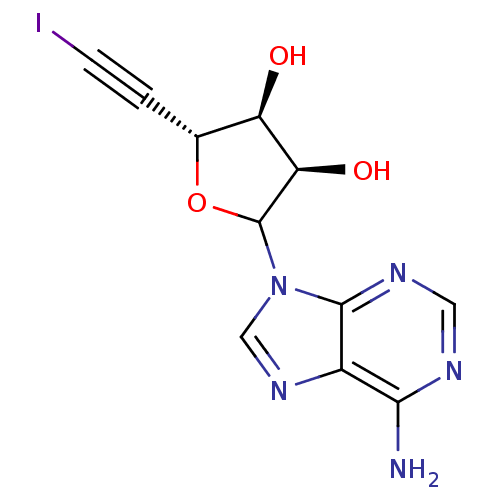
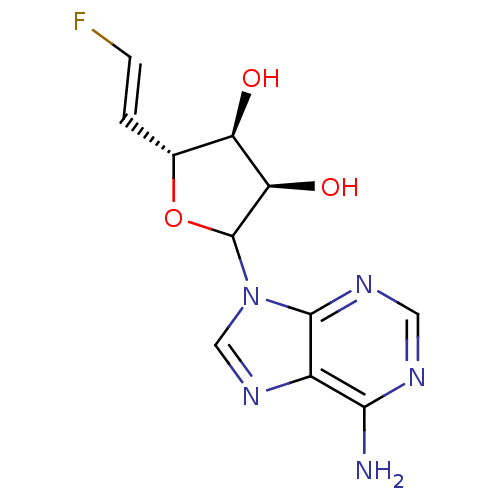

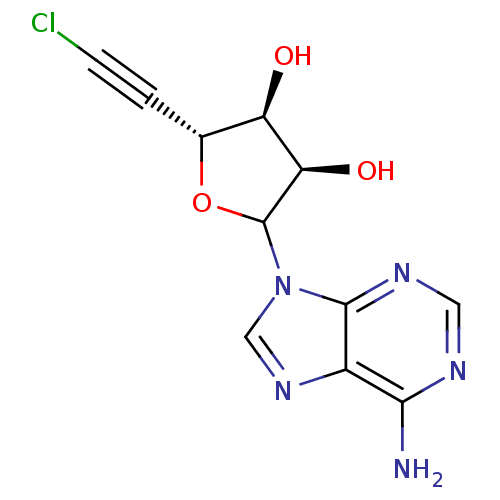
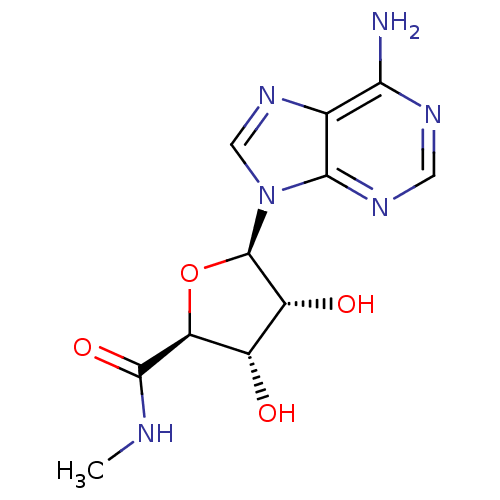
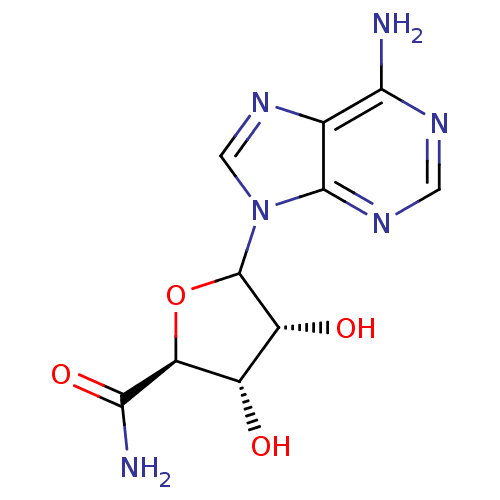
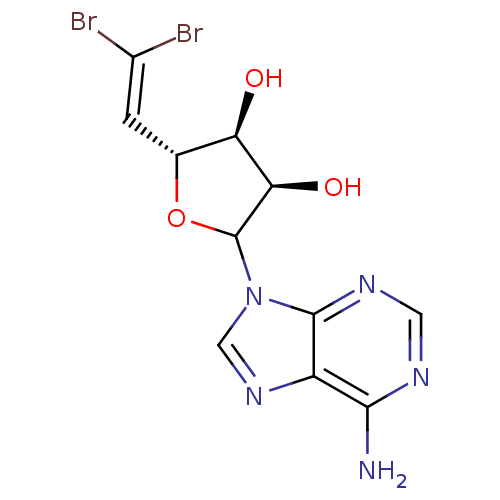

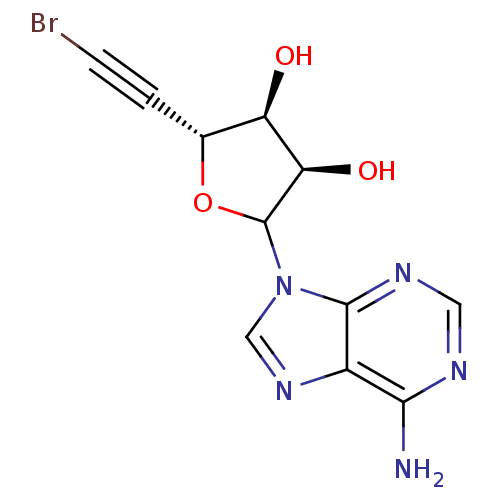
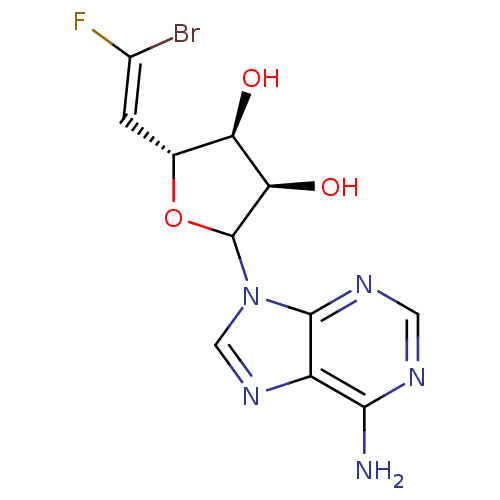
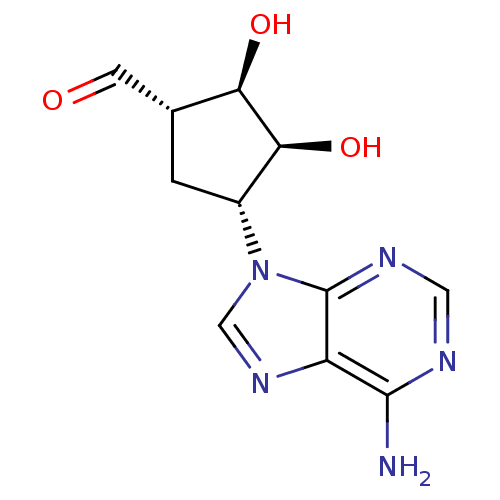
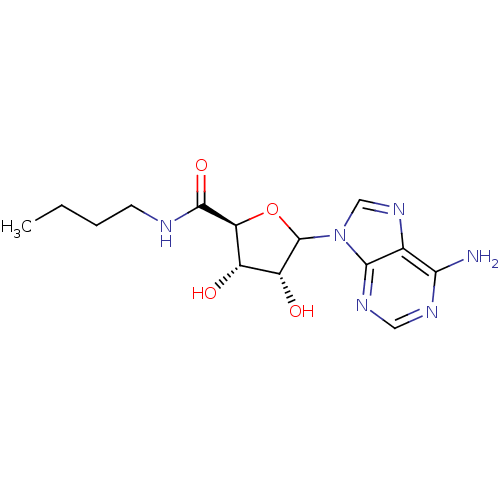
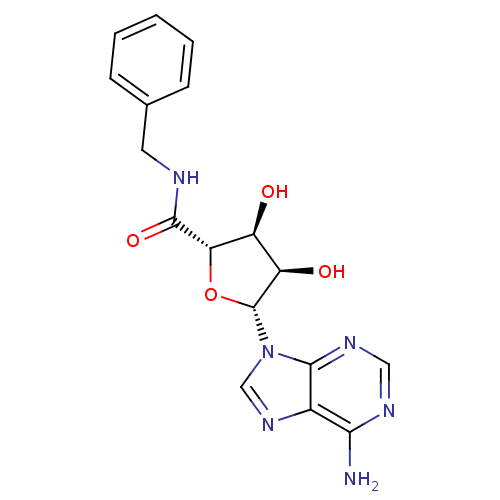
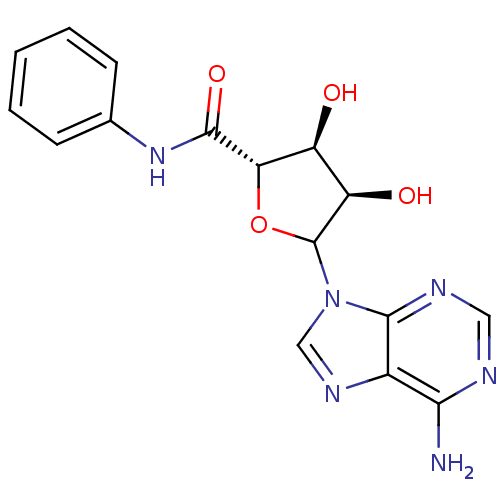

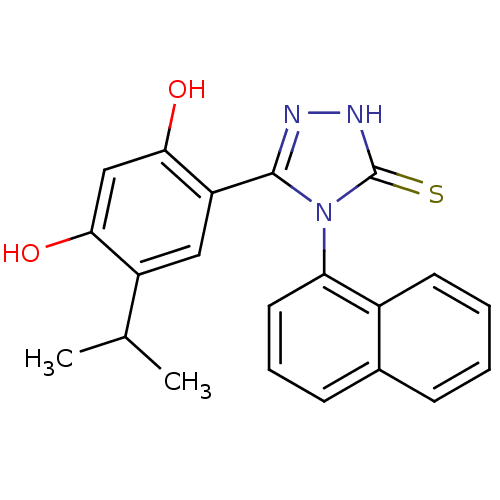
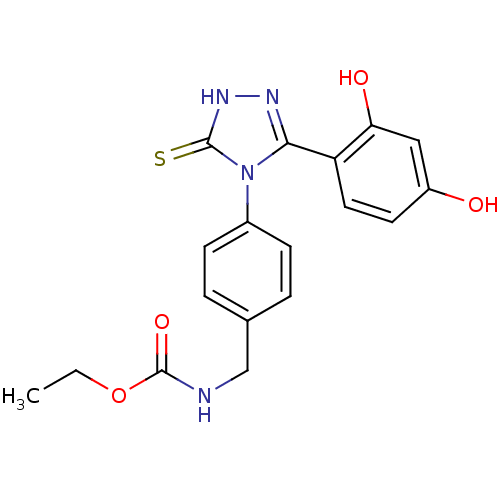

 3D Structure (crystal)
3D Structure (crystal)