 BDBM50420241 CHEMBL447221 ASPIRIN
BDBM50420241 CHEMBL447221 ASPIRIN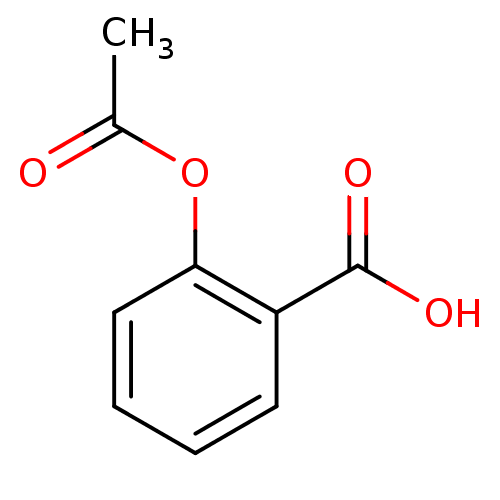 2-(acetyloxy)benzoate Zorprin Aspirin Endosprin Polopirin cid_2244 2-(acetyloxy)benzoic acid Acetylsalicylic acid BDBM22360 acetylsalicylate
2-(acetyloxy)benzoate Zorprin Aspirin Endosprin Polopirin cid_2244 2-(acetyloxy)benzoic acid Acetylsalicylic acid BDBM22360 acetylsalicylate
- Alagha, A; Moman, E; Adamo, MF; Nolan, KB; Chubb, AJ Design, synthesis and evaluation of aspirin analogues having an additional carboxylate substituent for antithrombotic activity. Bioorg Med Chem Lett 19: 4213-6 (2009)
- Lazzarato, L; Cena, C; Rolando, B; Marini, E; Lolli, ML; Guglielmo, S; Guaita, E; Morini, G; Coruzzi, G; Fruttero, R; Gasco, A Searching for new NO-donor aspirin-like molecules: Furoxanylacyl derivatives of salicylic acid and related furazans. Bioorg Med Chem 19: 5852-60 (2011)
- Abdellatif, KR; Chowdhury, MA; Dong, Y; Das, D; Yu, G; Velázquez, CA; Suresh, MR; Knaus, EE Dinitroglyceryl and diazen-1-ium-1,2-diolated nitric oxide donor ester prodrugs of aspirin, indomethacin and ibuprofen: synthesis, biological evaluation and nitric oxide release studies. Bioorg Med Chem Lett 19: 3014-8 (2009)
- ChEBML_157994 Inhibitory activity (RA2) against Prostaglandin G/H synthase 2 was calculated relative to aspirin
- ChEBML_159404 Inhibitory activity (RA1) against Prostaglandin G/H synthase 1 was calculated relative to aspirin
- ChEMBL_157994 (CHEMBL766667) Inhibitory activity (RA2) against Prostaglandin G/H synthase 2 was calculated relative to aspirin
- ChEMBL_159404 (CHEMBL763457) Inhibitory activity (RA1) against Prostaglandin G/H synthase 1 was calculated relative to aspirin
- COX1 and COX2 Inhibition Assay Approximately 2 µg of either COX-1 or COX-2 was added to buffer containing 100 µM AA, 0.1 M Tris-HCl buffer (pH 8.0), 5 mM EDTA, 2 mM phenol, and 1 µM hematin at 37 °C.Inhibitors were incubated with the respective COX isozyme for 20 min and added to the reaction mixture, and the rate of oxygen consumption was recorded. Ibuprofen, aspirin, and the carrier solvent, DMSO, were used as positive and negative controls, respectively

