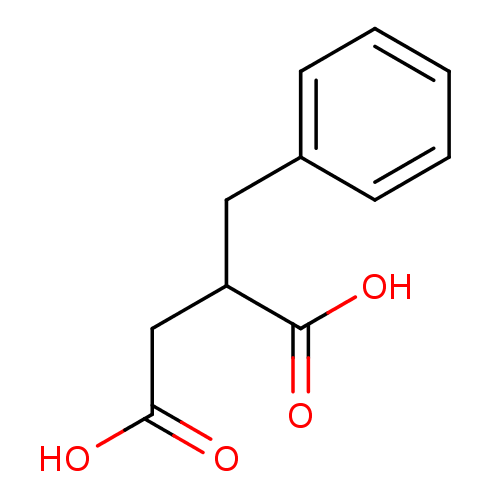
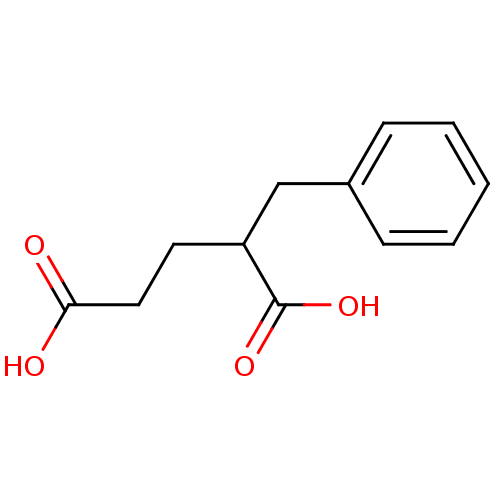
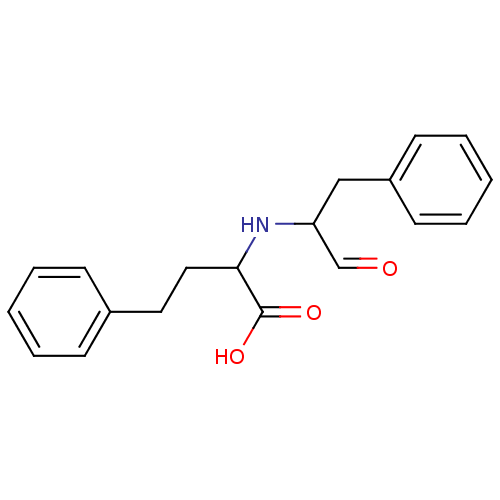
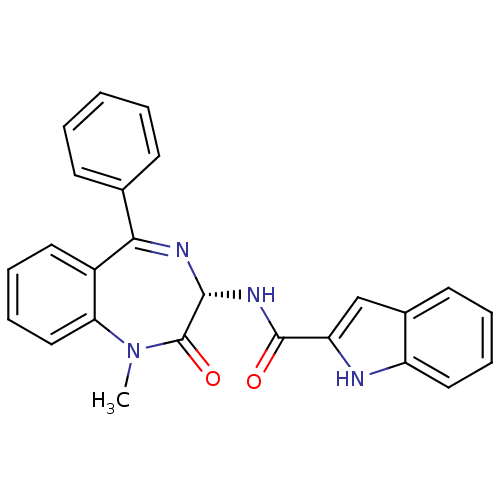
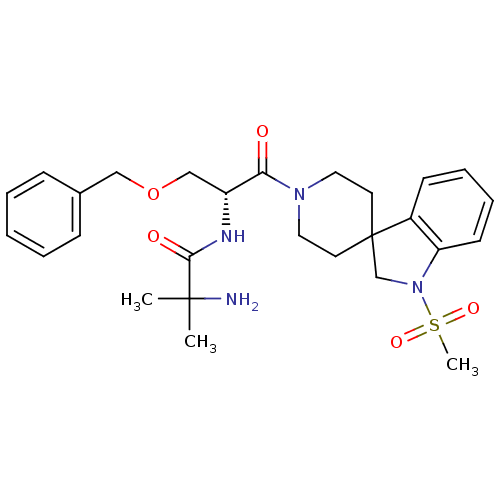
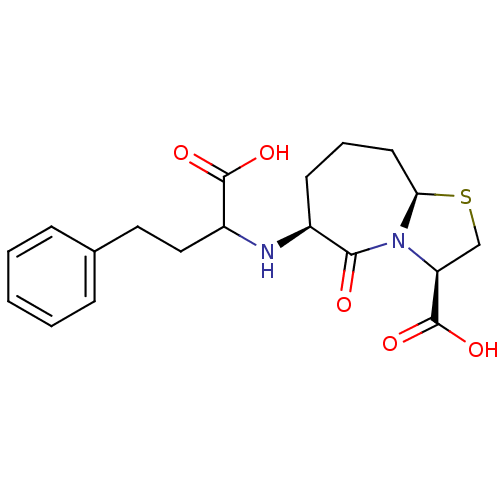
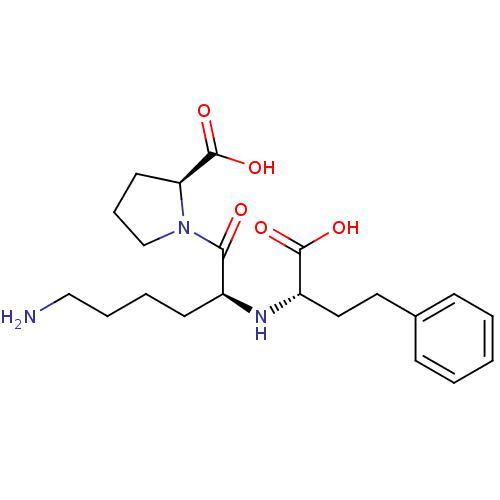
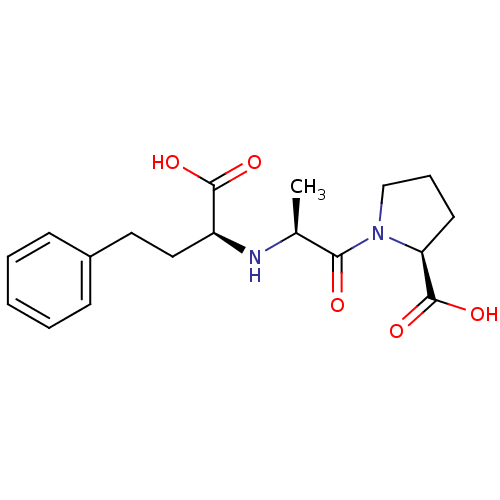
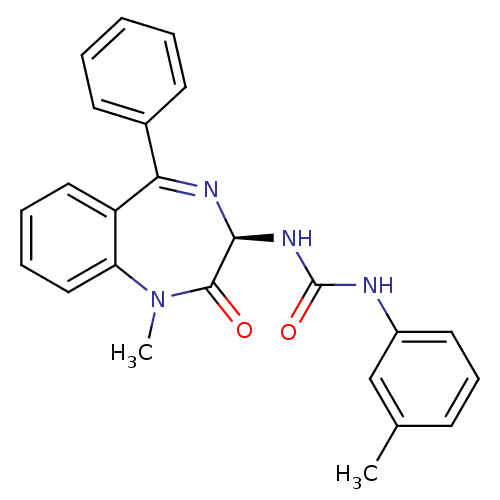
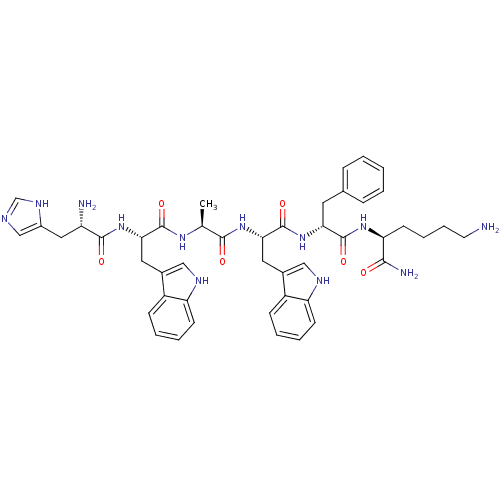
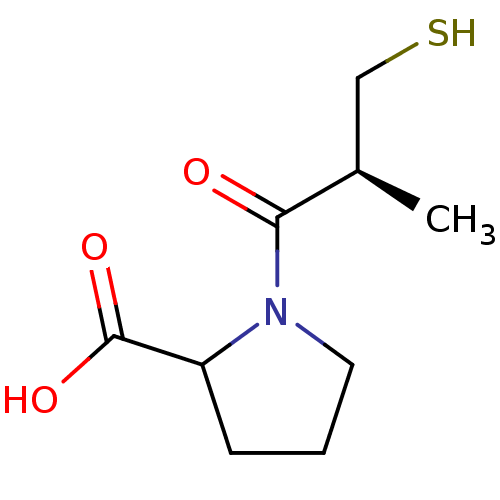
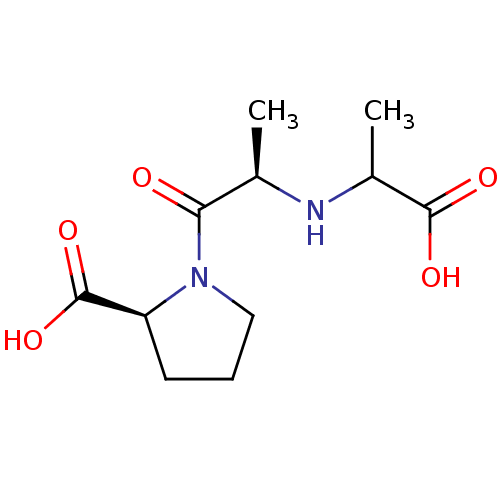
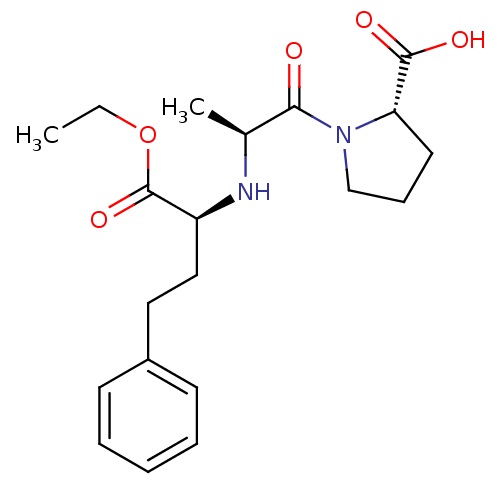
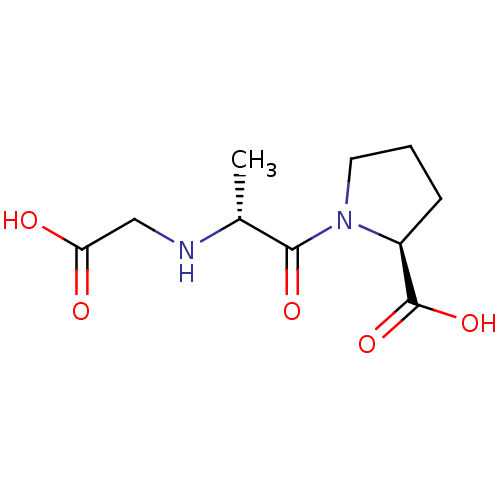
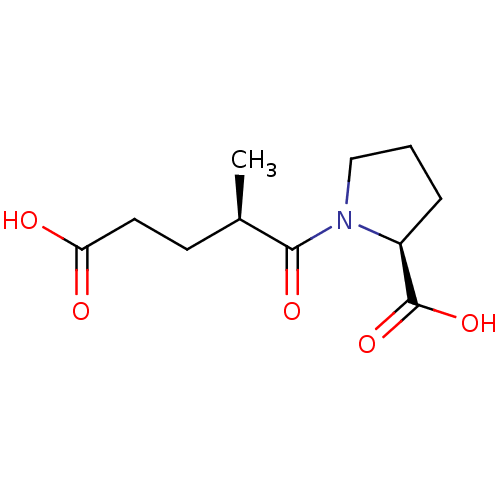
TargetSomatostatin receptor type 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 0.0100nMAssay Description:Binding affinity towards Somatostatin receptor type 2 (hsst2)More data for this Ligand-Target Pair
TargetSomatostatin receptor type 3(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 31nMAssay Description:Binding affinity towards Somatostatin receptor type 3 (hsst3)More data for this Ligand-Target Pair
TargetSomatostatin receptor type 4(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 81nMAssay Description:Binding affinity towards Somatostatin receptor type 4 (hsst4)More data for this Ligand-Target Pair
TargetSomatostatin receptor type 5(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 163nMAssay Description:Binding affinity towards Somatostatin receptor type 5 (hsst5)More data for this Ligand-Target Pair
TargetMast cell carboxypeptidase A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 450nMAssay Description:The compound was evaluated for the inhibition of Carboxypeptidase AMore data for this Ligand-Target Pair
TargetSomatostatin receptor type 1(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.39E+3nMAssay Description:Binding affinity towards Somatostatin receptor type 1 (hsst1)More data for this Ligand-Target Pair
TargetMast cell carboxypeptidase A(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMAssay Description:The compound was evaluated for the inhibition of Carboxypeptidase AMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 5.00E+4nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) in venom of Bothrops jararacaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0800nMAssay Description:Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissueMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.510nMAssay Description:Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) receptor expressed in COS-7 cellsMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 0.710nMAssay Description:Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) expressed in COS-7 cellsMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of Cholecystokinin type B receptor induced guinea pig gall bladder contractions when given intravenouslyMore data for this Ligand-Target Pair
TargetGrowth hormone secretagogue receptor type 1(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 13.2nMAssay Description:Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) expressed in COS-7 cellsMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 23nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 90nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 245nMAssay Description:Inhibition of Cholecystokinin type B receptor induced guinea pig gall bladder contractions when given intravenouslyMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissueMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Concentration required for 50% inhibition of Cholecystokinin type A receptor in rat pancreatic tissueMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.90E+3nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.00E+4nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of Cholecystokinin type B receptor induced guinea pig gall bladder contractions when given intravenouslyMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.60E+5nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair


 3D Structure (crystal)
3D Structure (crystal)