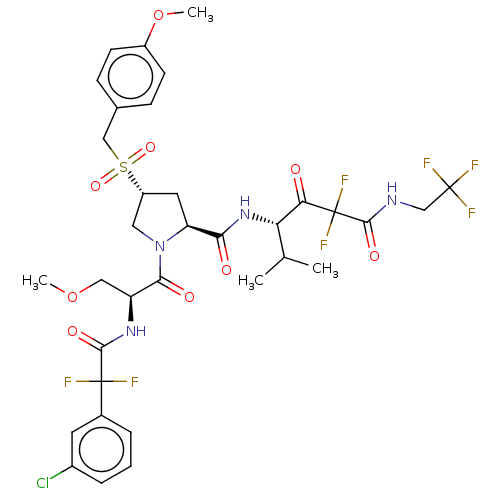
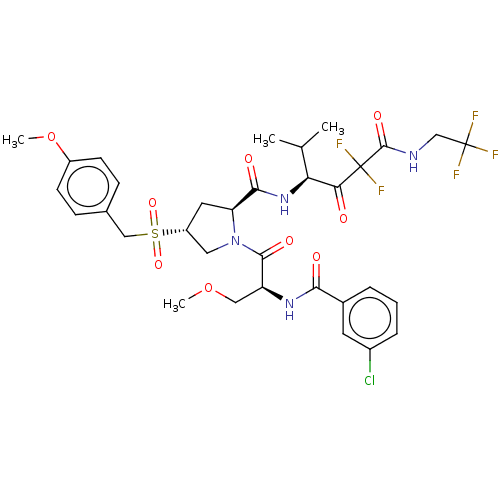
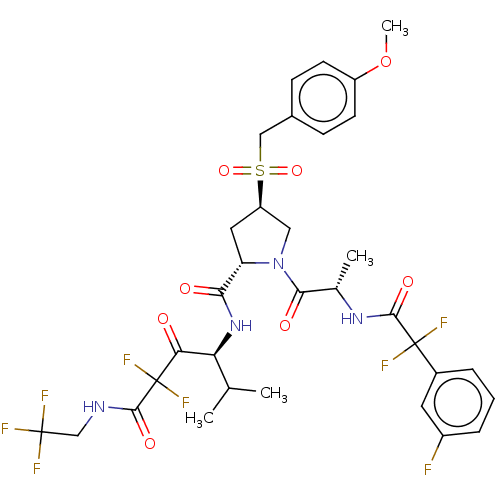
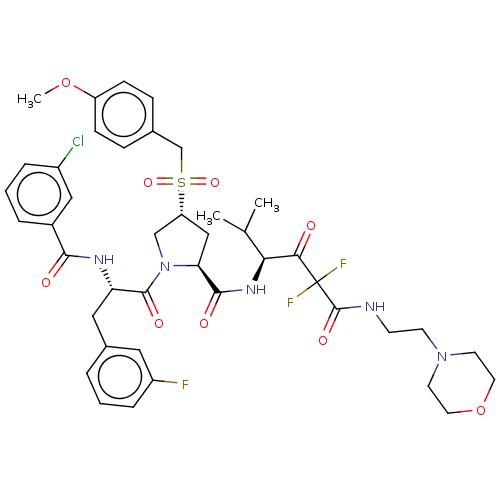
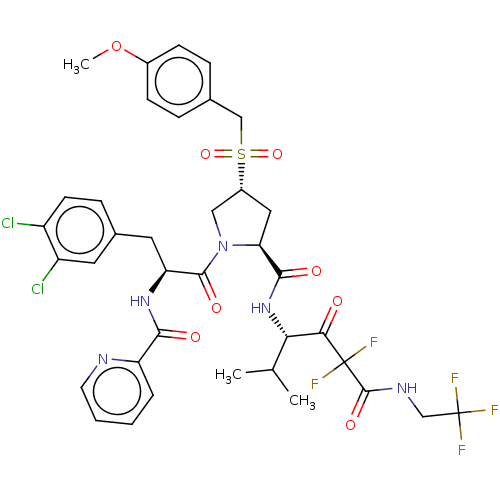
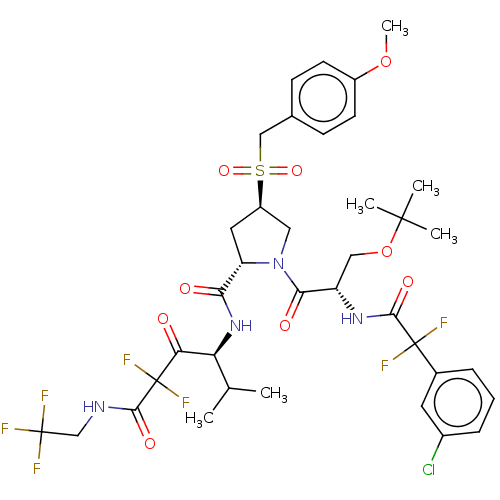
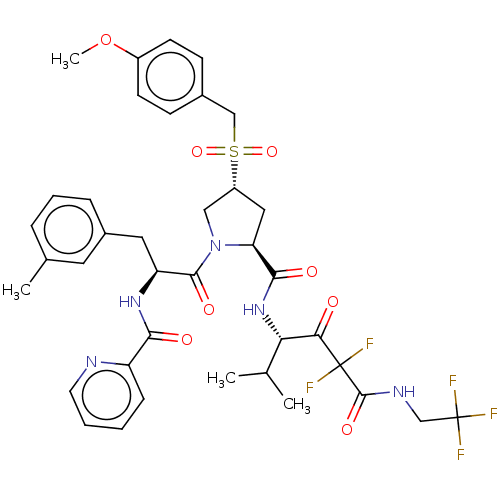
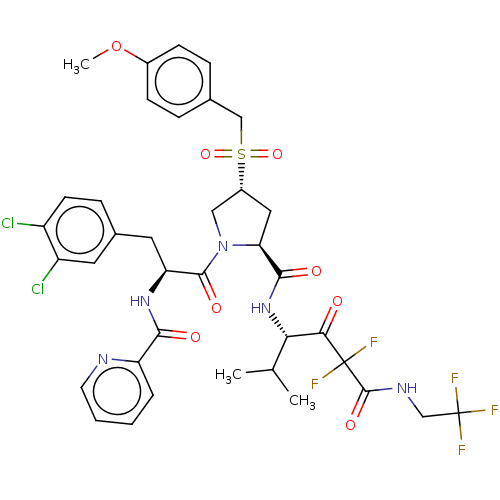
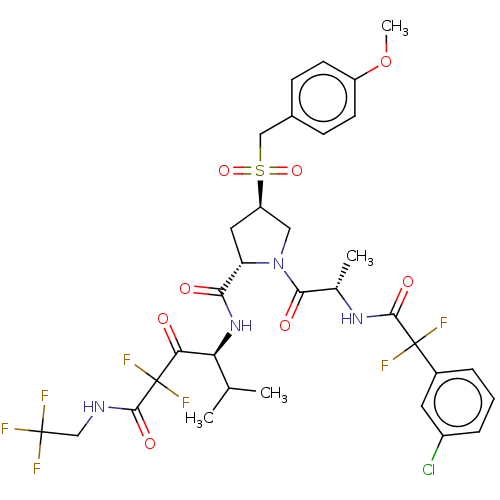
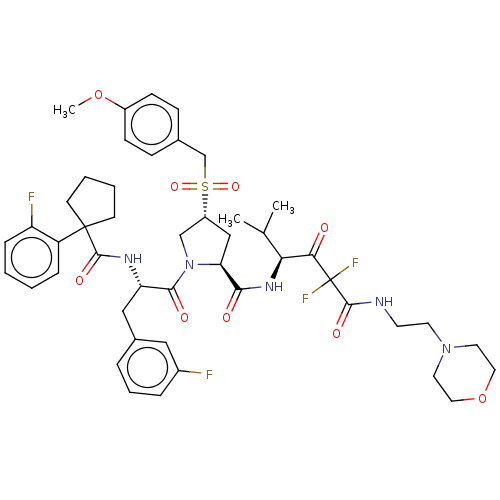
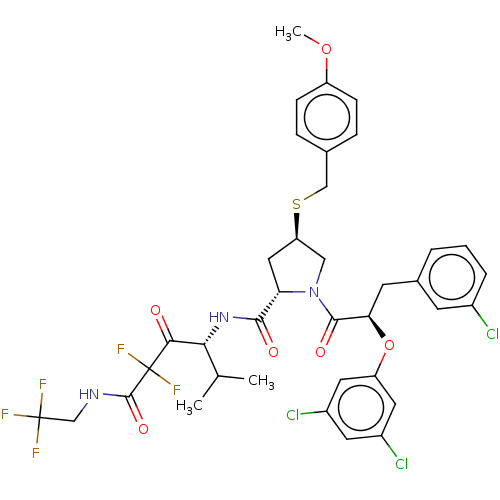
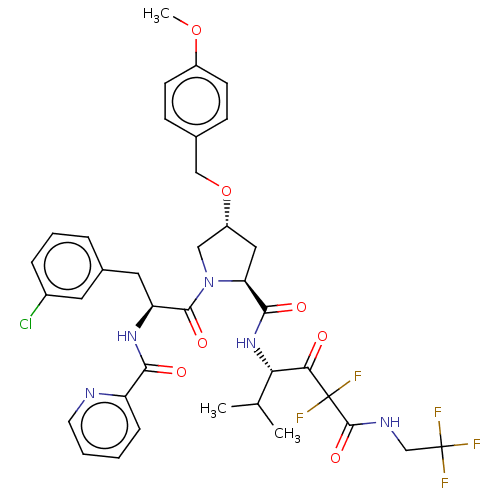
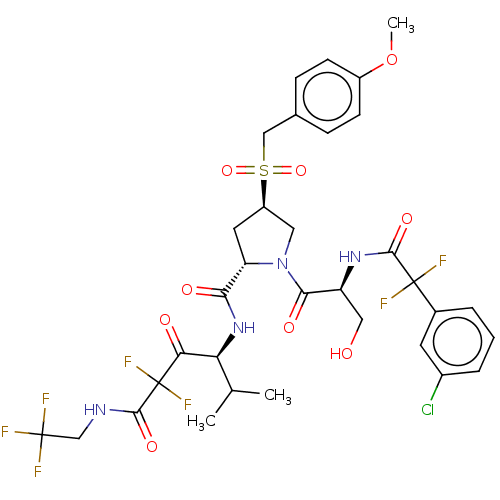
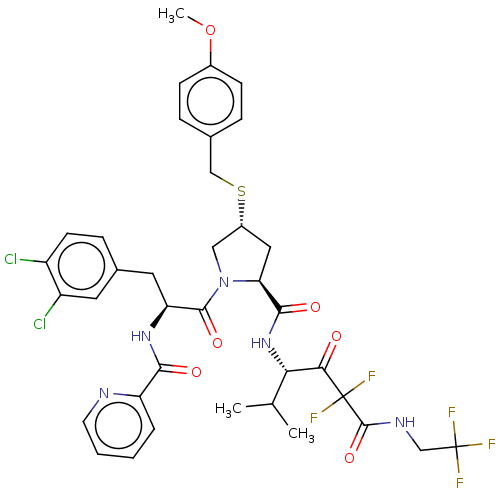
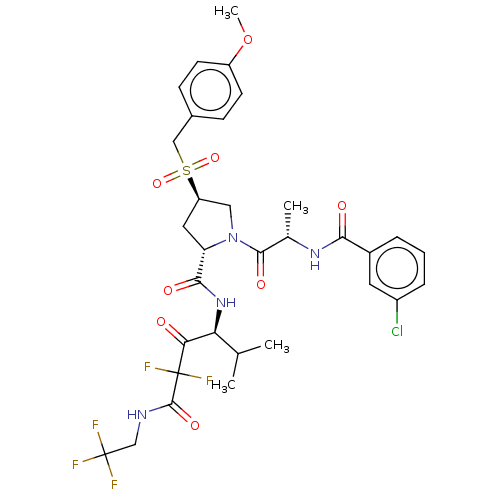
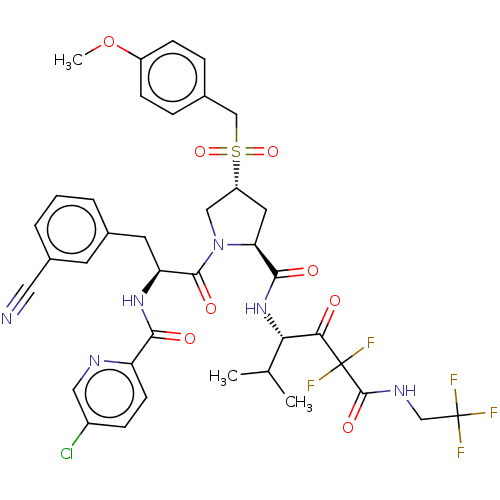
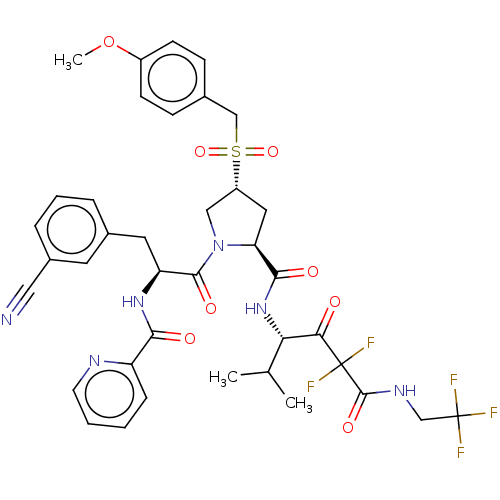
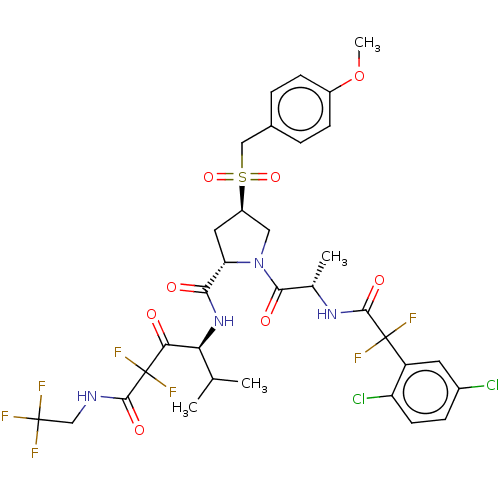
Affinity DataIC50: 0.262nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.351nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.471nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.538nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.574nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.579nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.667nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.694nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.781nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.804nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.838nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.871nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.876nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.889nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.897nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.933nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.06nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.14nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.25nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.31nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.33nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 2.08nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair