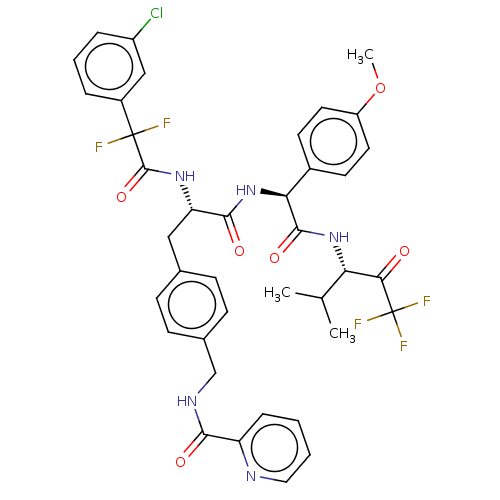
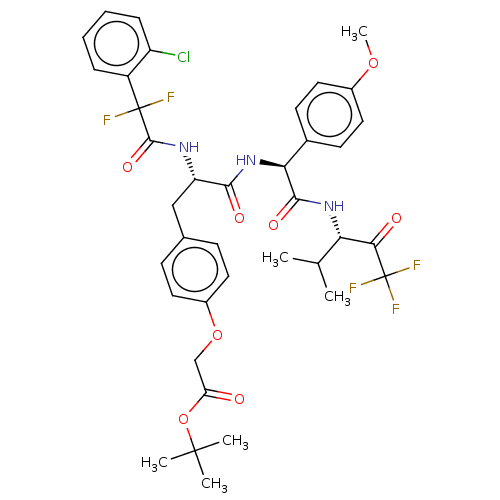
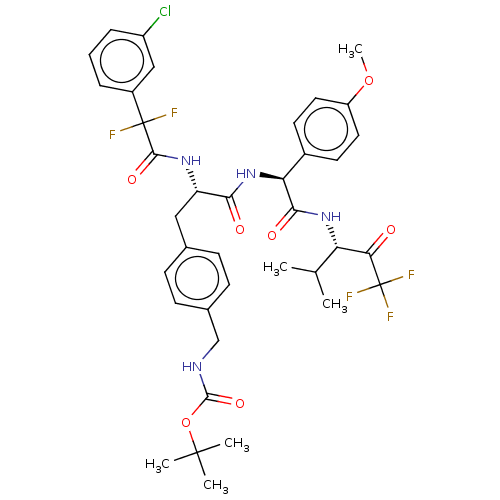
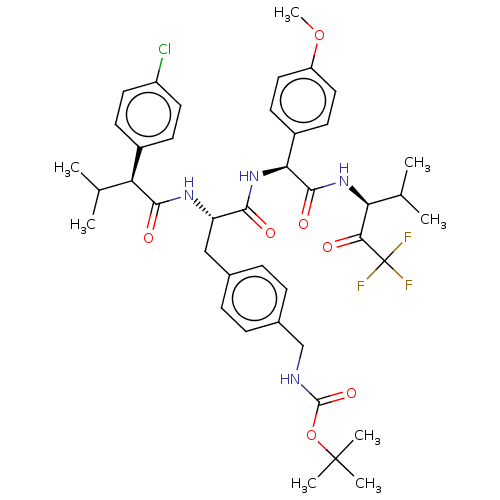
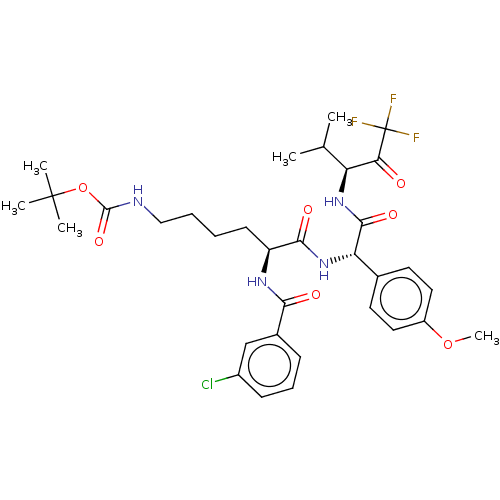
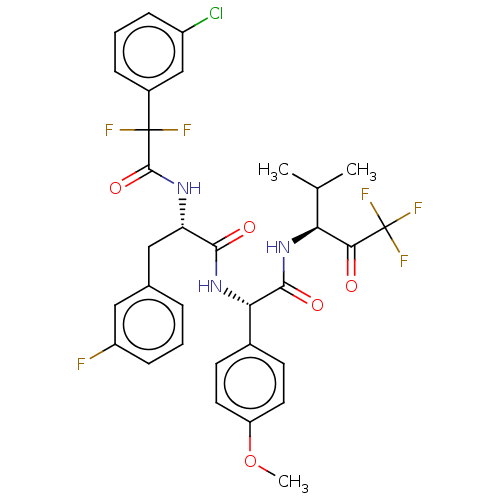
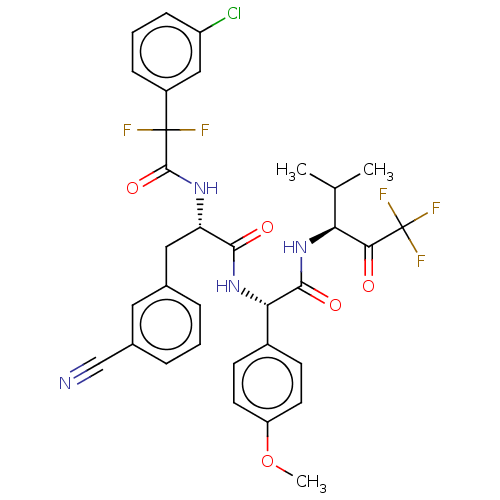
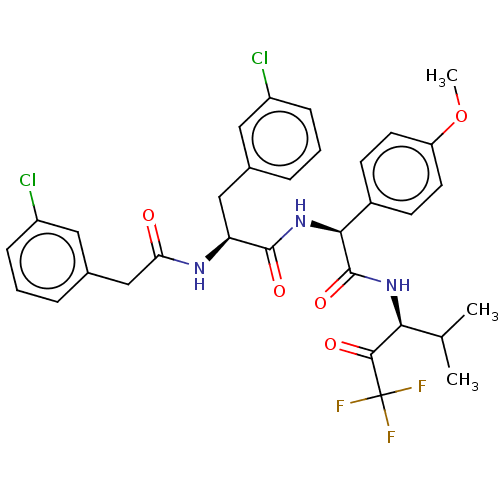
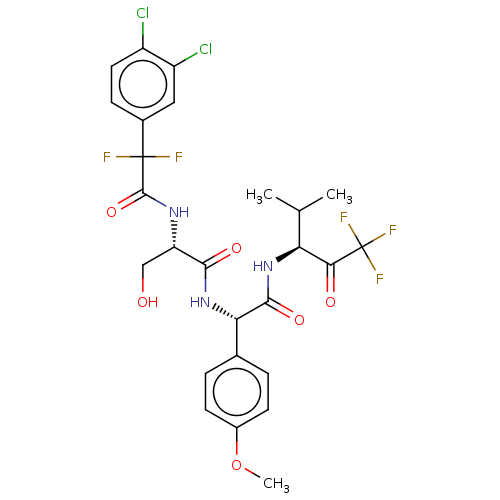
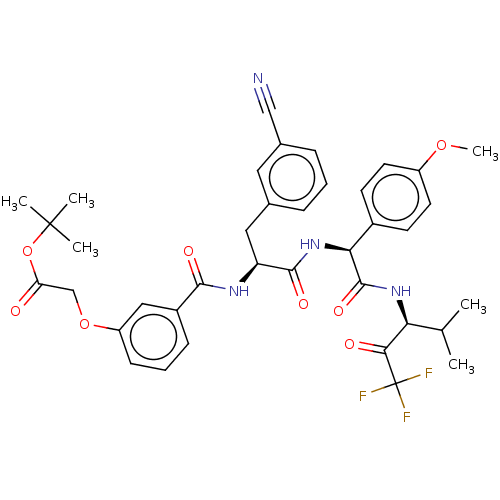
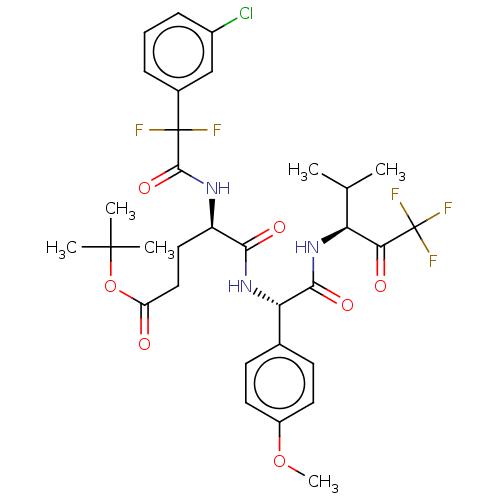
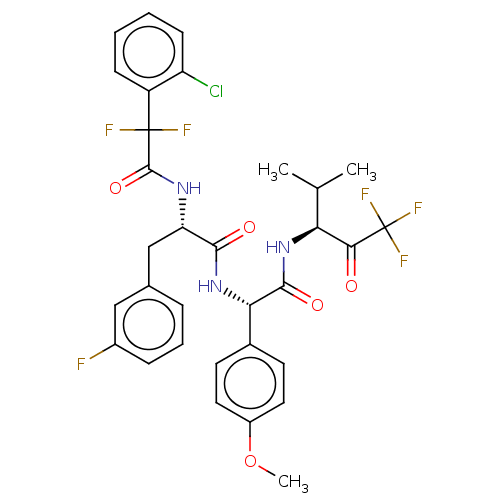
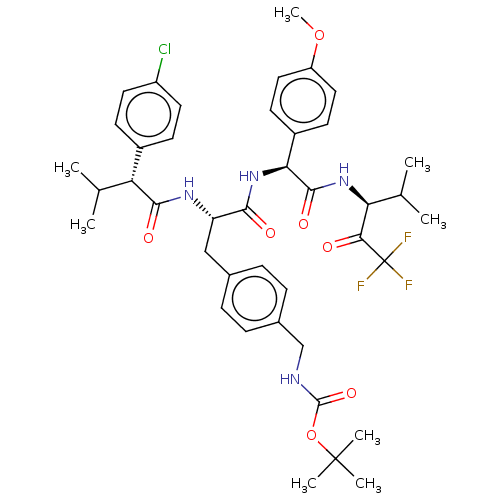
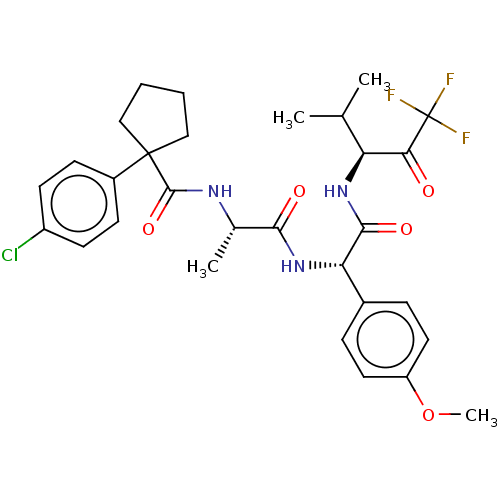
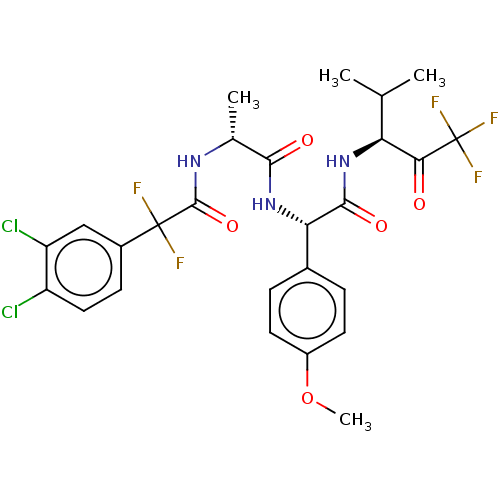
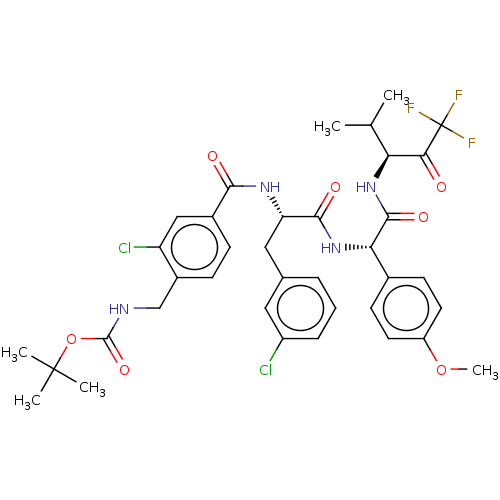
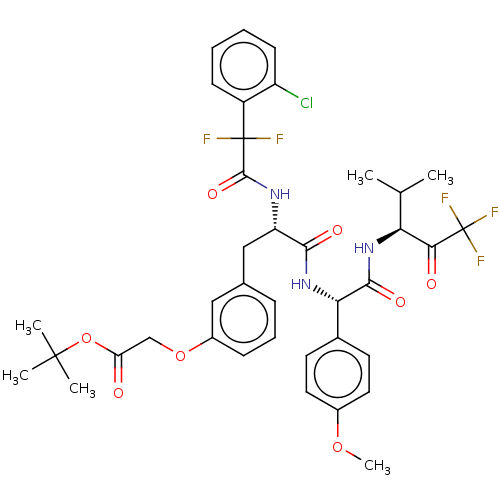
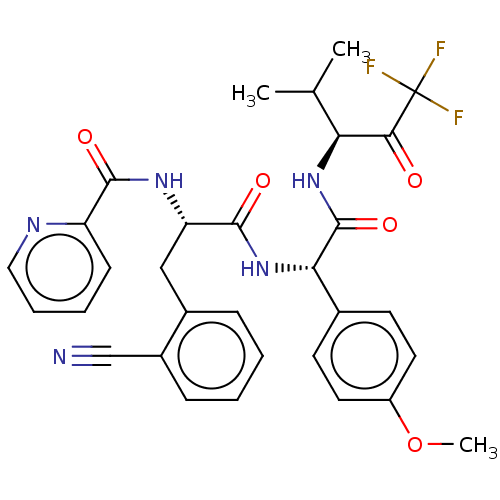
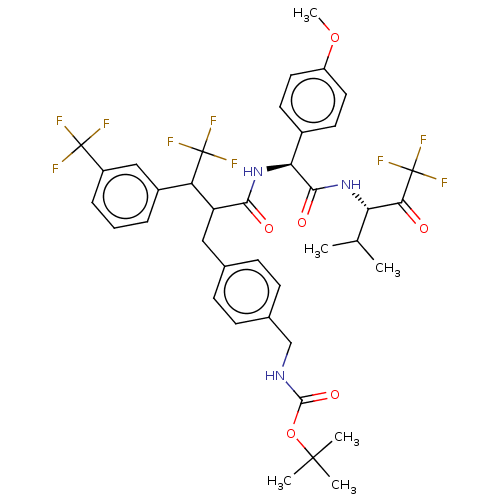
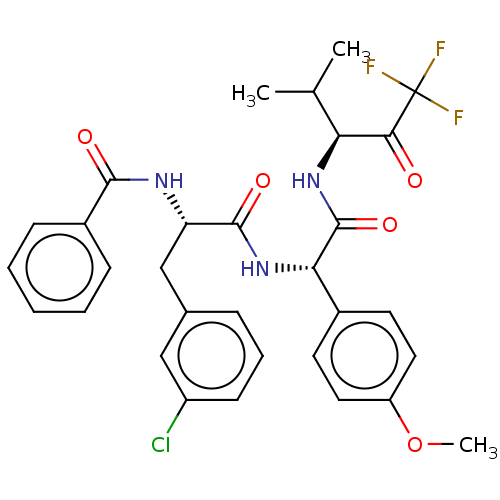
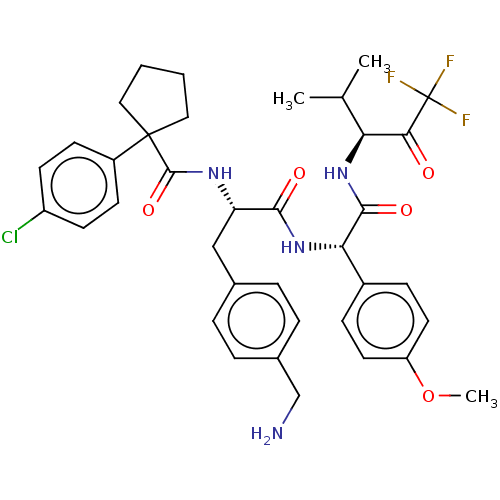
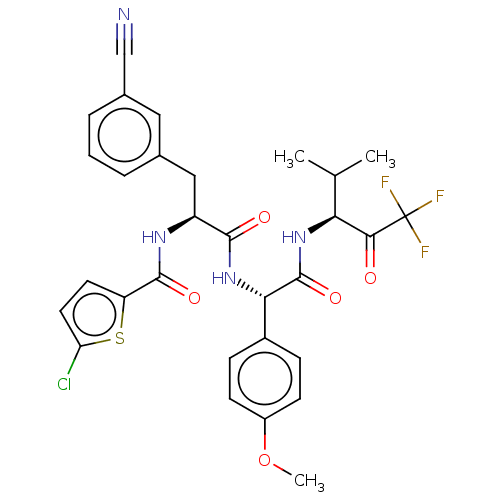
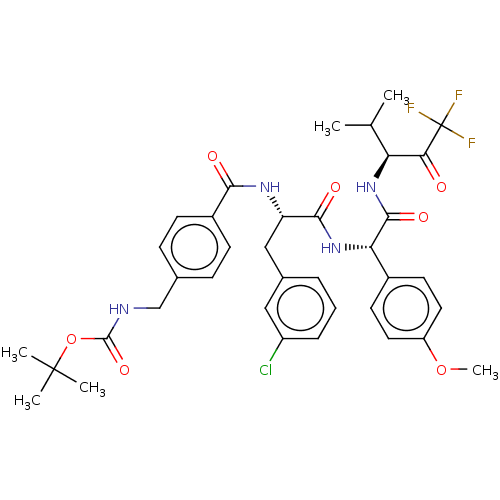
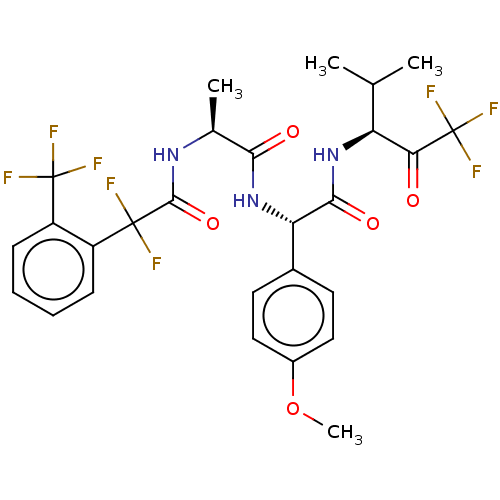
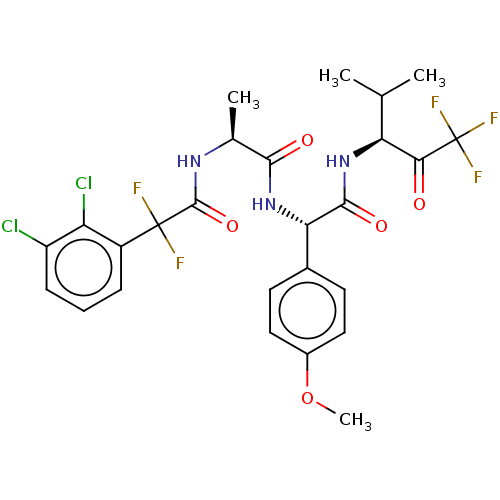
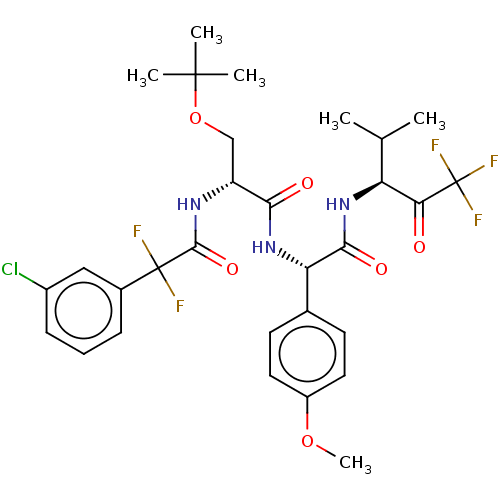
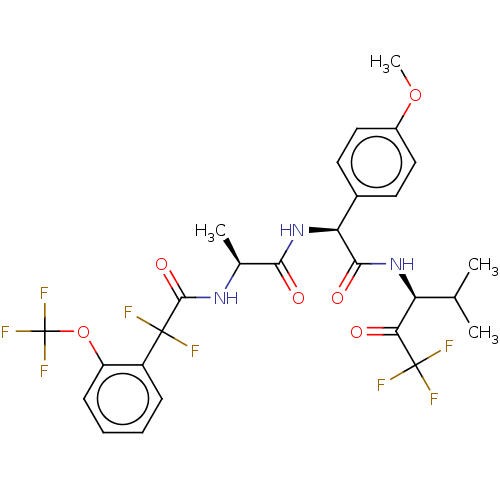
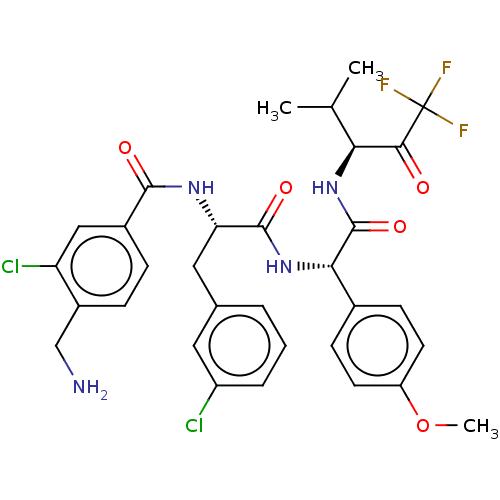
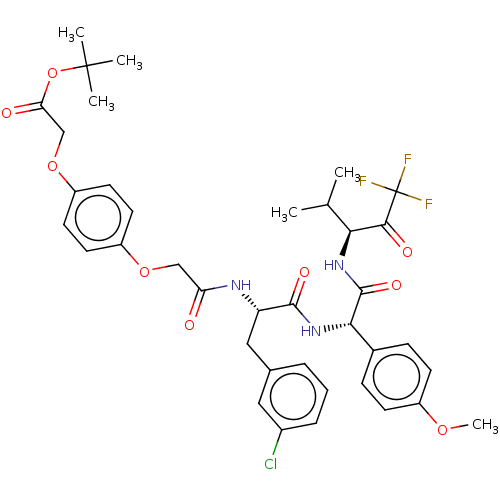
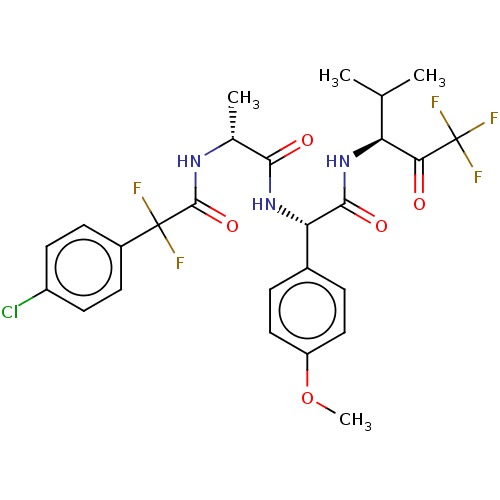
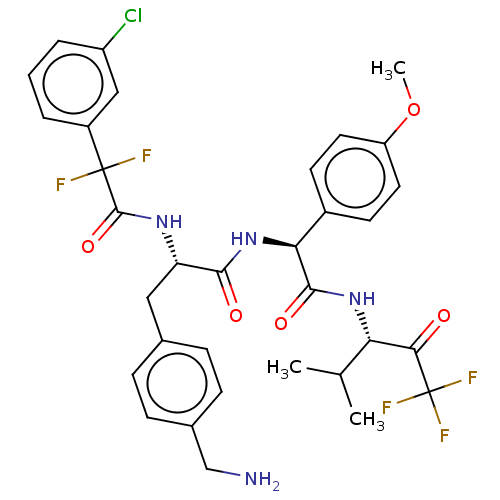
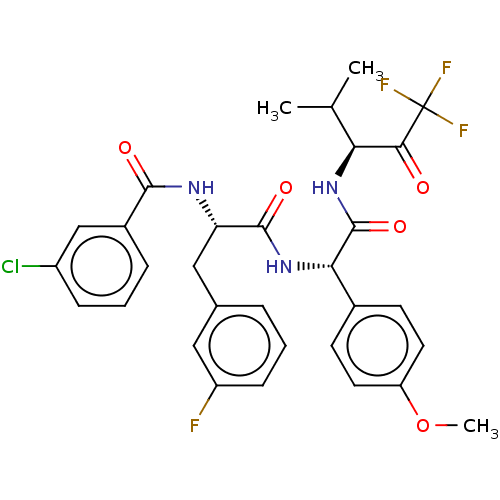
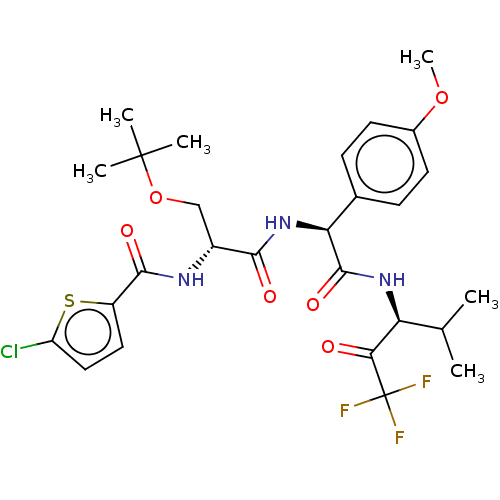
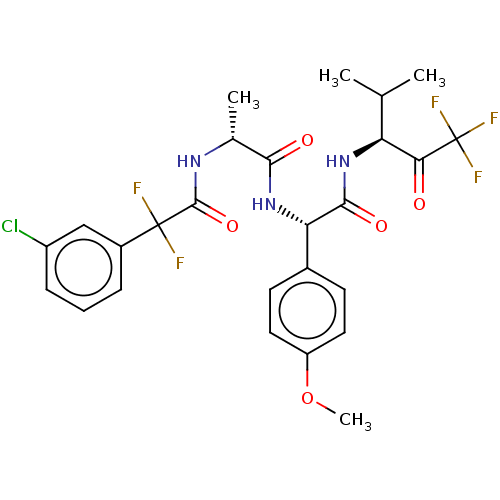
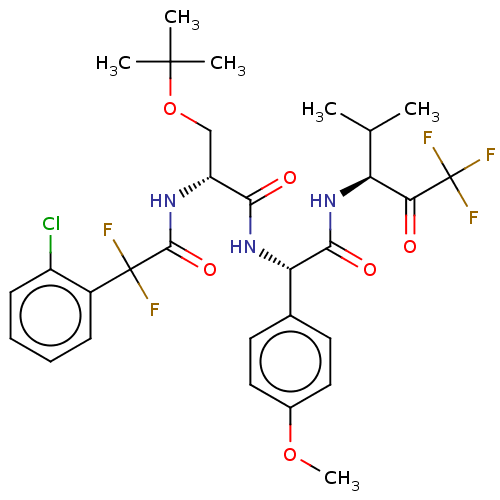
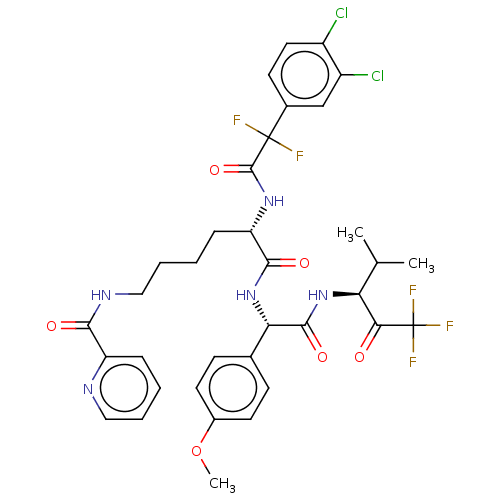
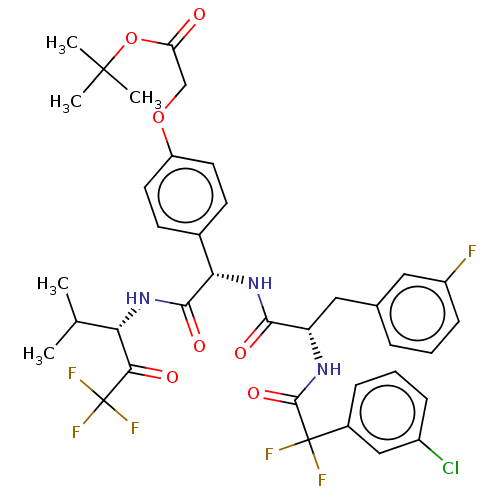
Affinity DataIC50: 0.400nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.490nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.602nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.695nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.760nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.830nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 0.970nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.10nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.30nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.82nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair