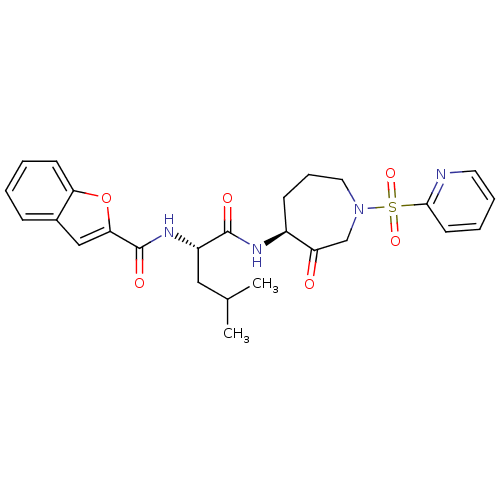
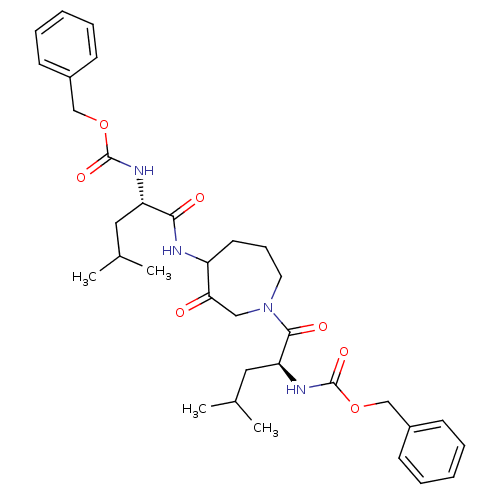
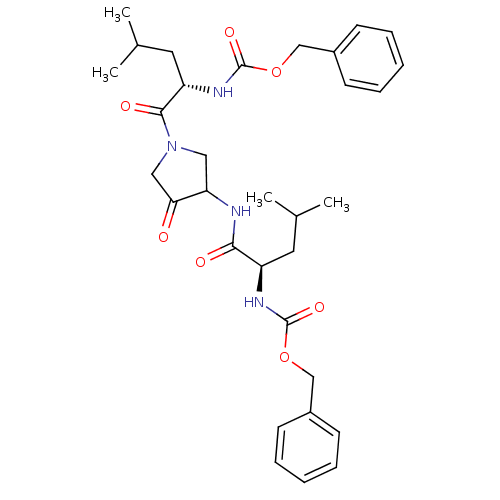
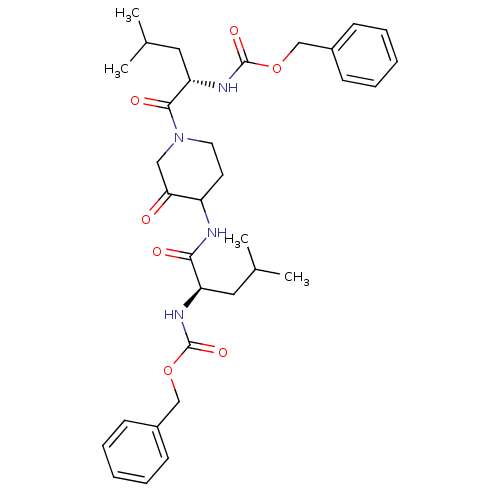
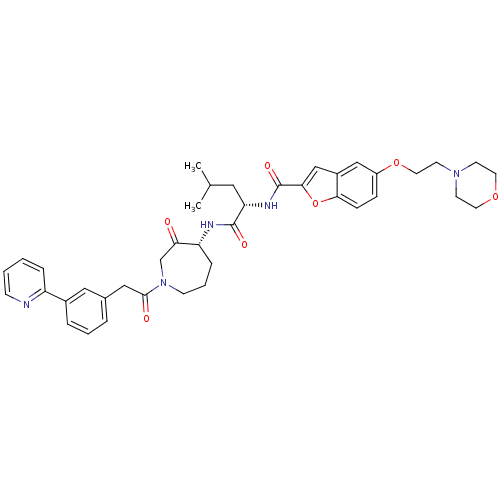
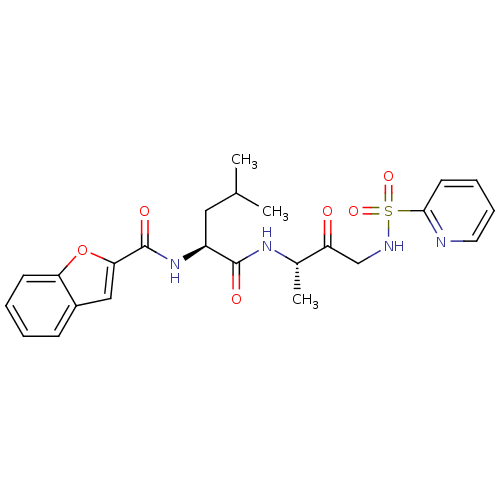
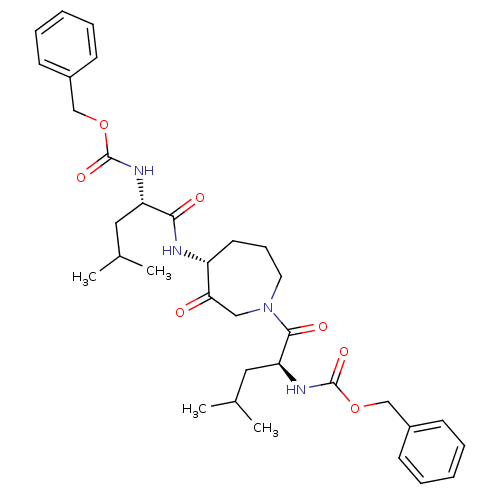
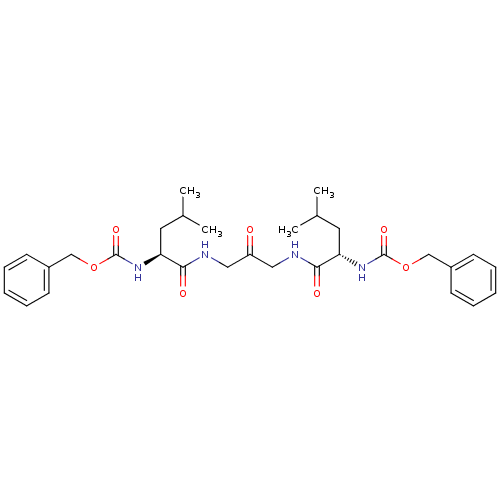
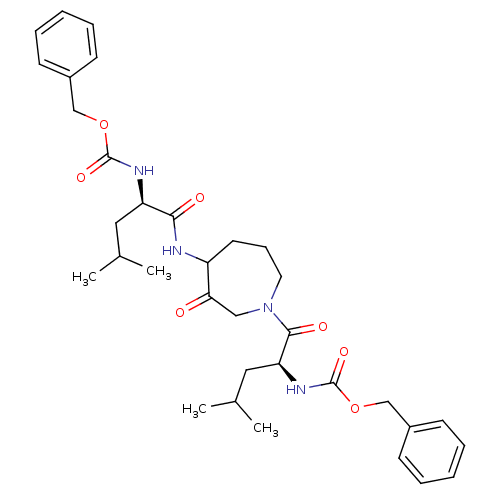
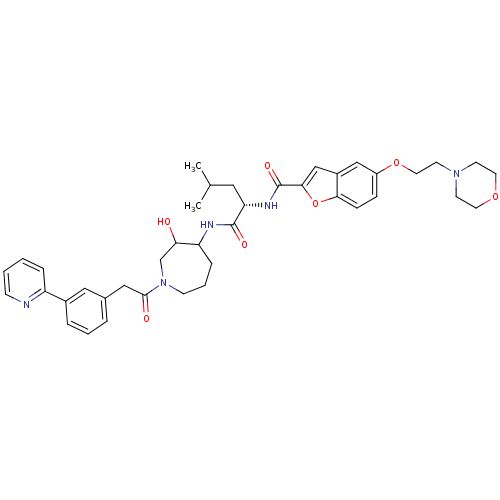
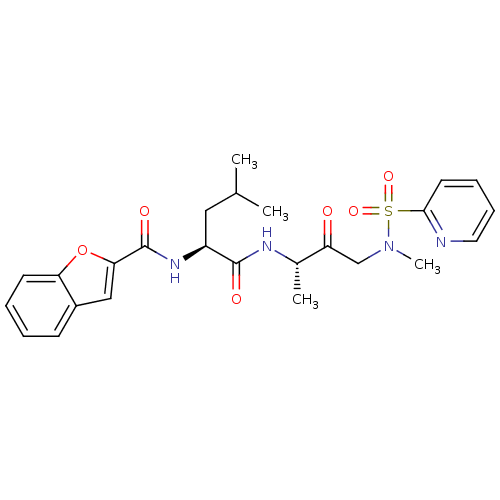
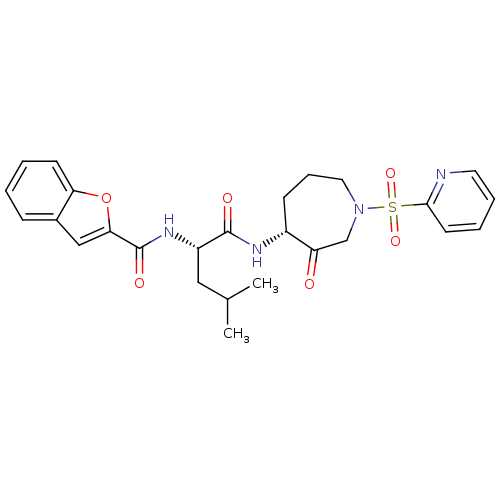
Affinity DataKi: 0.00480nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibitory activity of the compound against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibitory activity of the compound against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 97nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 340nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 440nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 670nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 890nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 980nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro cell based assay for measuring the IC50 values of osteoblast resorption.More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro IC50 value of the compound was measured using in situ cytochem assay.More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:in vitro cell based assay for measuring the IC50 values of osteoblast resorption.More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:In vitro IC50 value of the compound was measured using in situ cytochem assay.More data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)