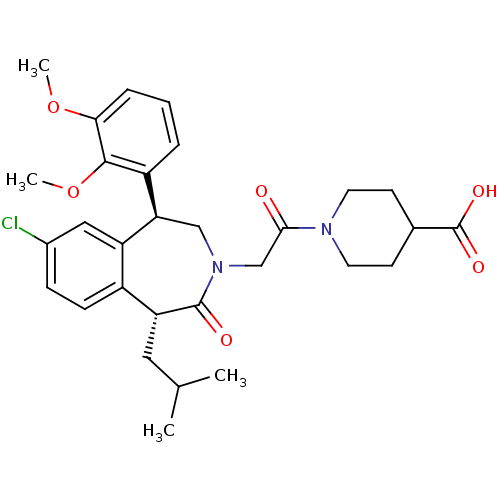
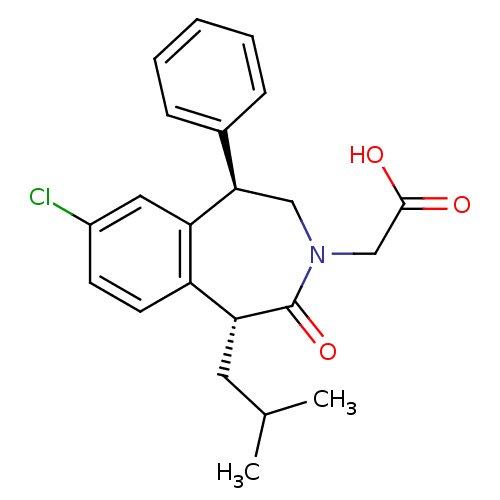
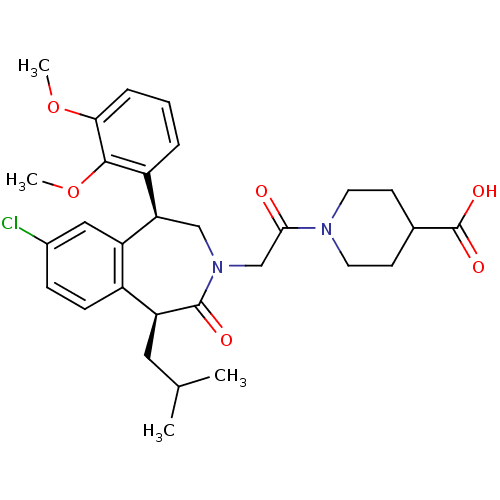
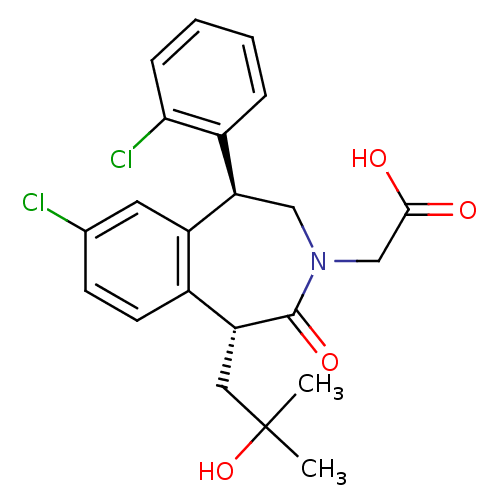
Affinity DataIC50: 220nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
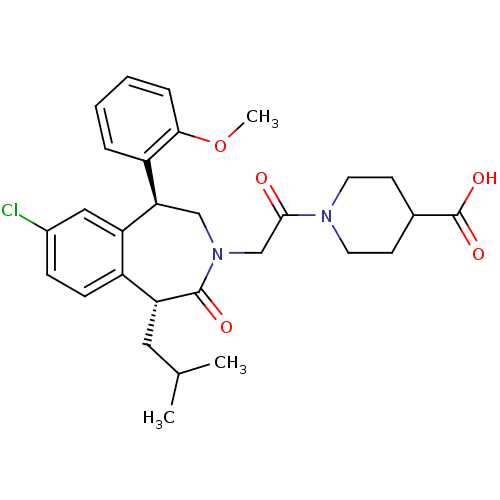
Affinity DataIC50: 370nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
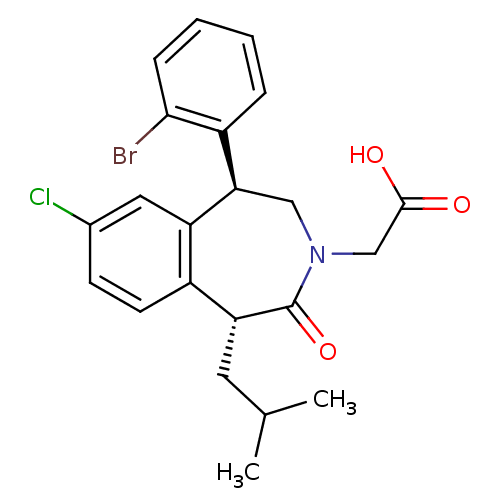
Affinity DataIC50: 450nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
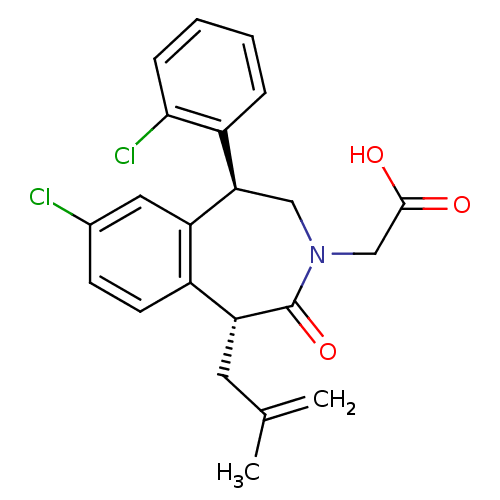
Affinity DataIC50: 500nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 930nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of squalene synthase assessed as conversion of [3H]farnesyl phosphate to [3H]squalene after 10 mins by scintillation countingMore data for this Ligand-Target Pair