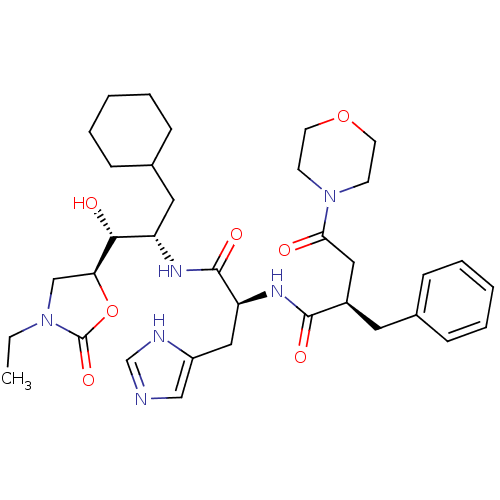
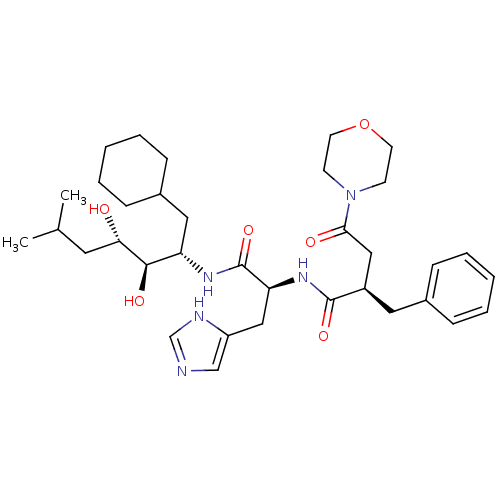
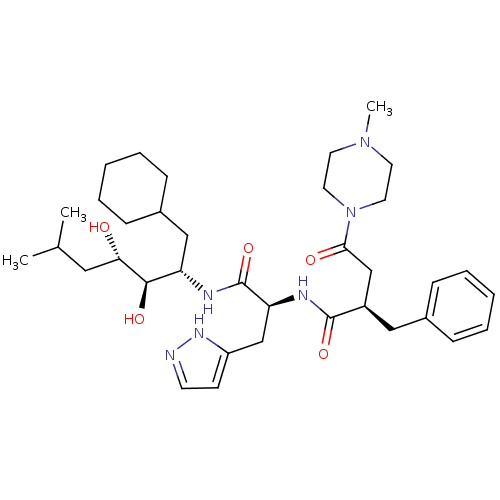
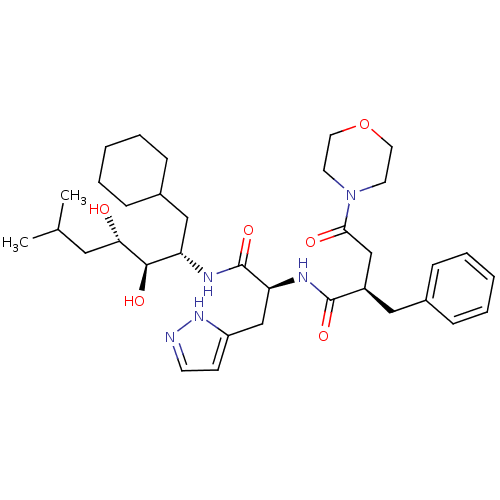
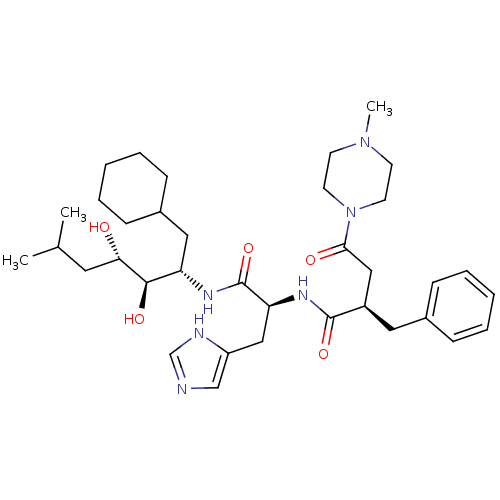
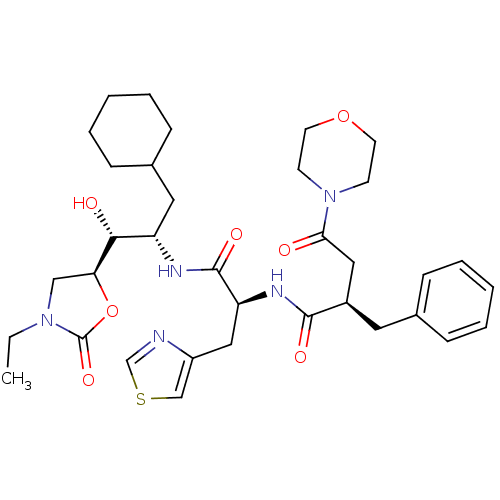
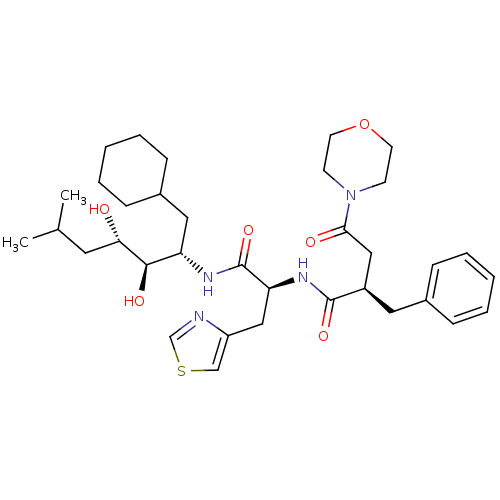
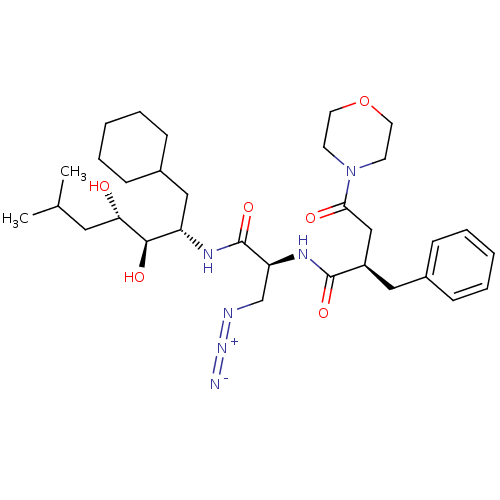
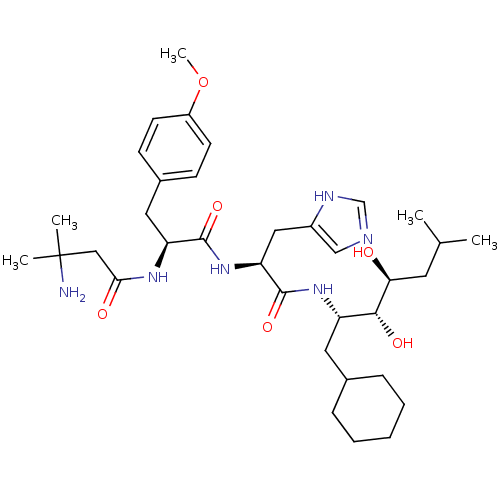
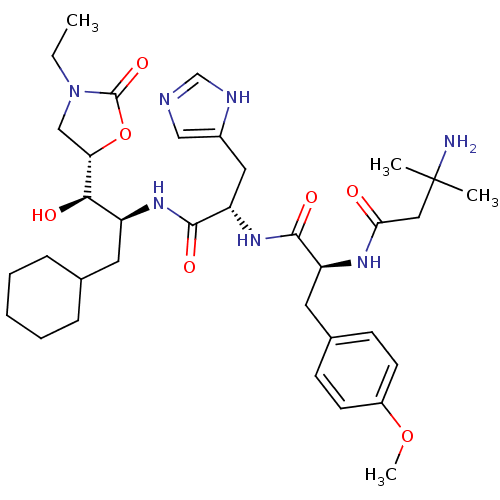
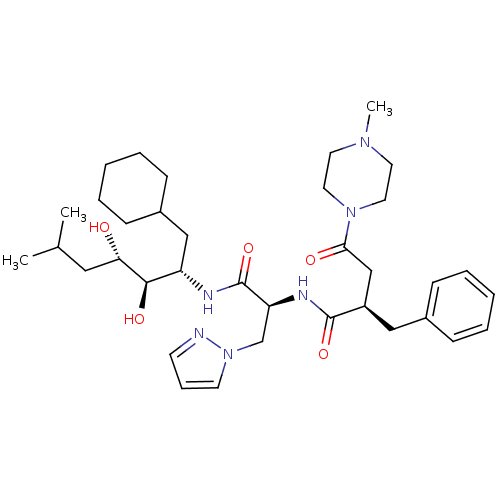
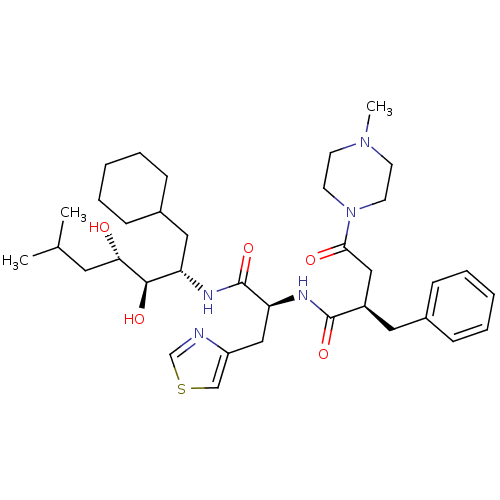
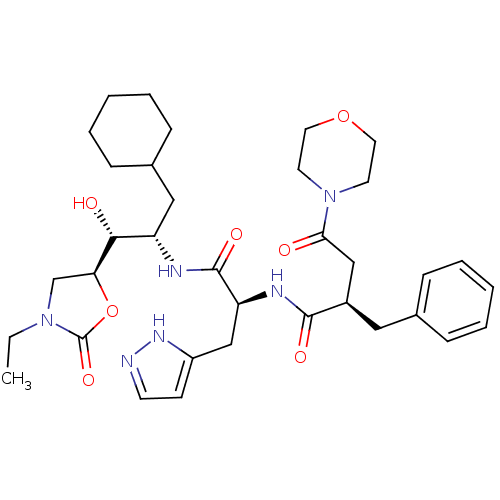
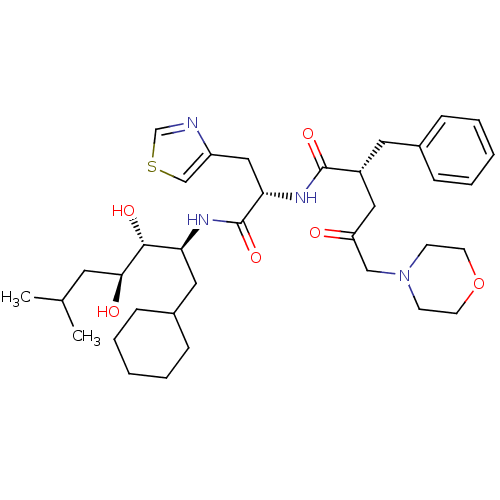
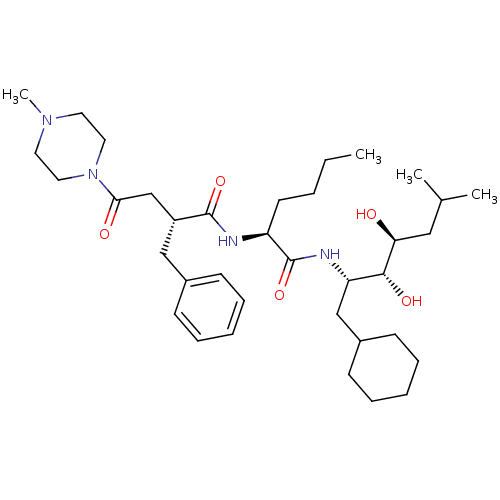
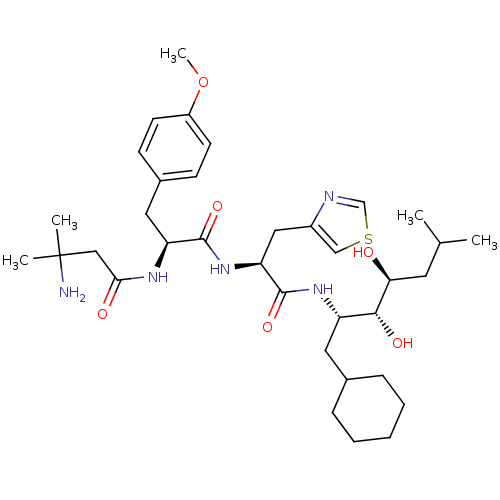
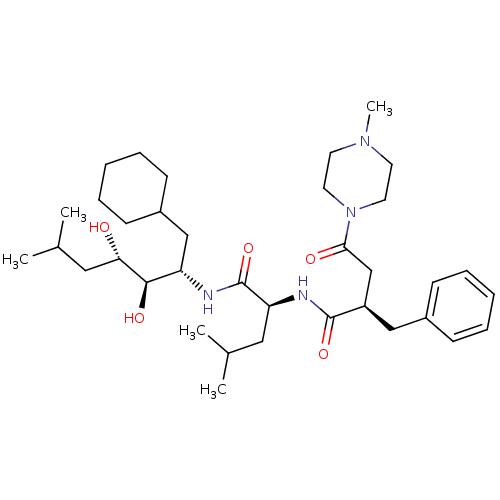
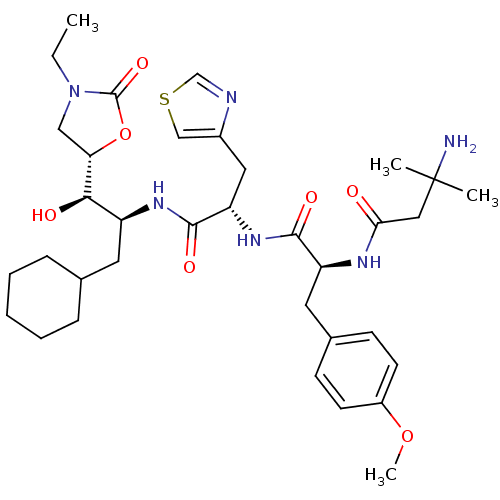
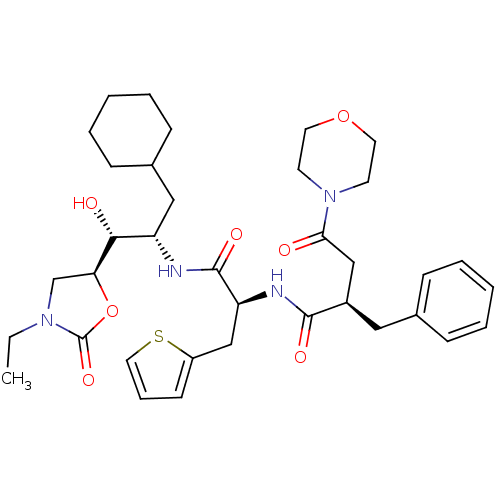
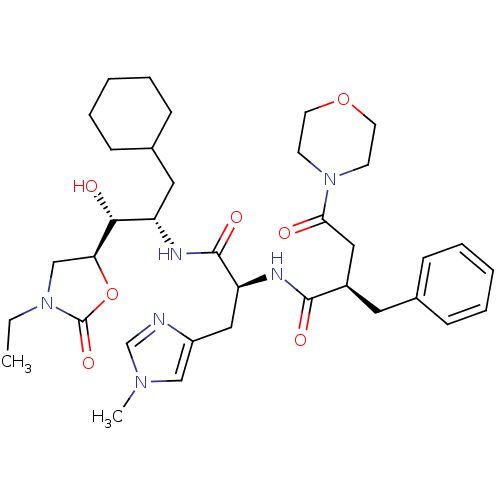
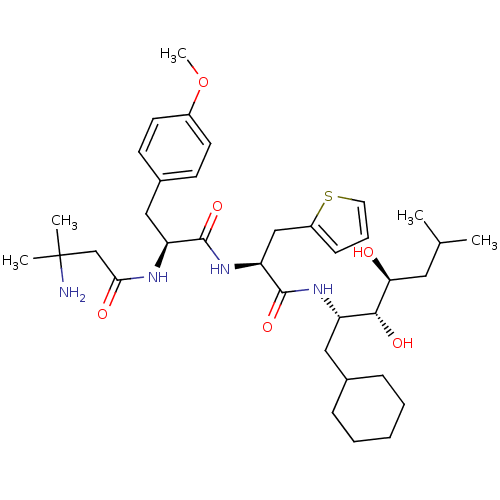
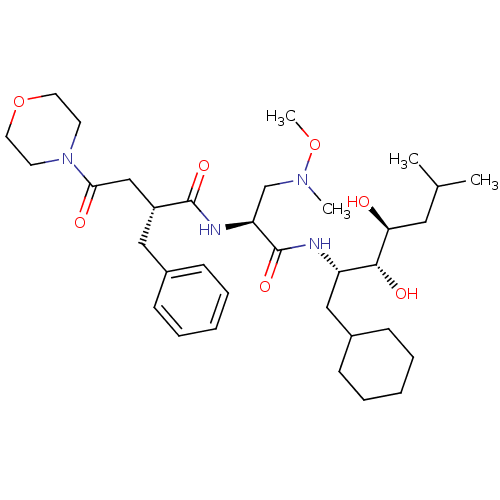
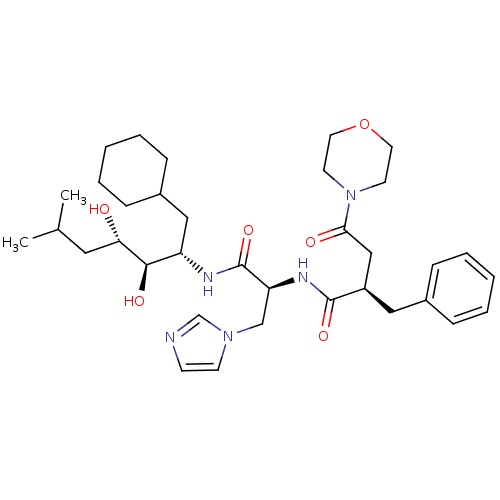
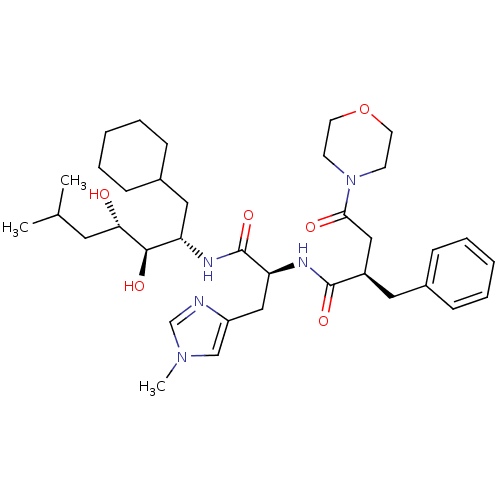
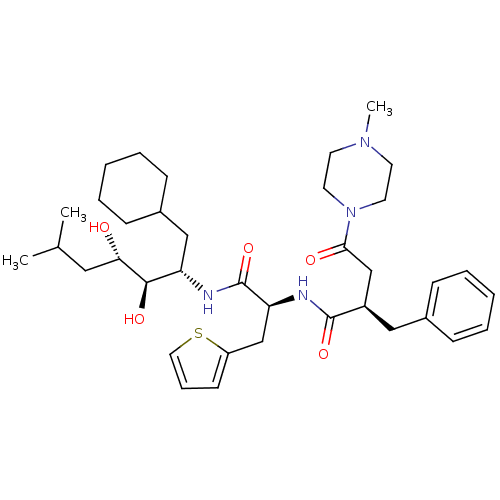
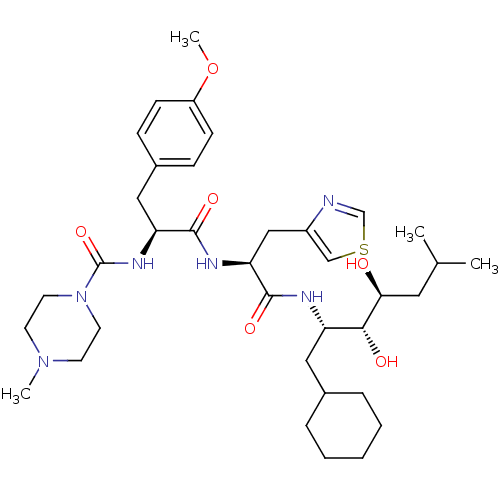
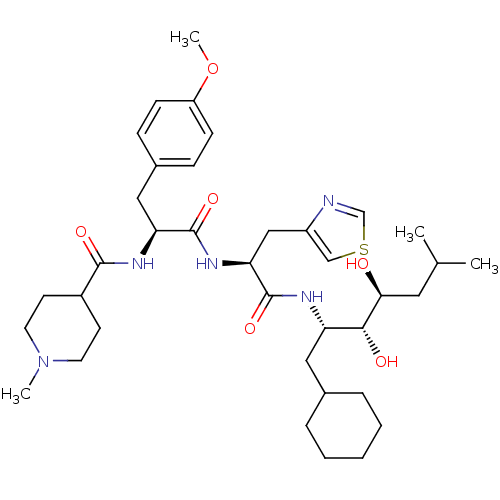
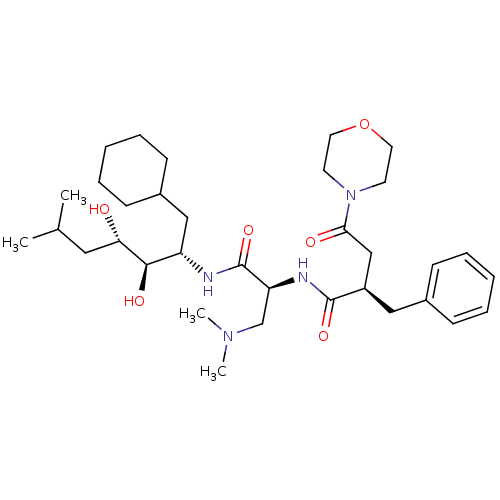
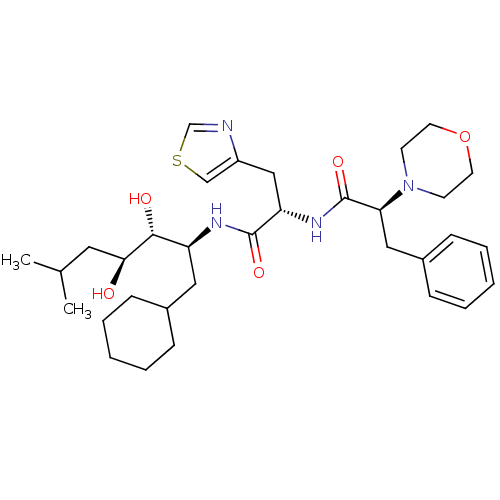
Affinity DataIC50: 1.30nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 3.5nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 5.40nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 8.10nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 8.30nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 11nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 14nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 17nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 18nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 18nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 21nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 25nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 33nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 37nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 39nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 43nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 58nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 69nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 85nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 88nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 93nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 110nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 110nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 120nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 170nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 210nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMpH: 7.4Assay Description:Inhibition of human plasma renin at pH 7.4More data for this Ligand-Target Pair