Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
S-adenosylmethionine decarboxylase proenzyme
Ligand
BDBM28437
Substrate
BDBM28422
Meas. Tech.
AdoMetDC Inhibition Assay
pH
6.8±n/a
Temperature
295.15±n/a K
IC50
1500±n/a nM
Citation
 McCloskey, DE; Bale, S; Secrist, JA; Tiwari, A; Moss, TH; Valiyaveettil, J; Brooks, WH; Guida, WC; Pegg, AE; Ealick, SE New Insights into the Design of Inhibitors of Human S-Adenosylmethionine Decarboxylase: Studies of Adenine C(8) Substitution in Structural Analogues of S-Adenosylmethionine (dagger). J Med Chem 52:1388-407 (2009) [PubMed] Article
McCloskey, DE; Bale, S; Secrist, JA; Tiwari, A; Moss, TH; Valiyaveettil, J; Brooks, WH; Guida, WC; Pegg, AE; Ealick, SE New Insights into the Design of Inhibitors of Human S-Adenosylmethionine Decarboxylase: Studies of Adenine C(8) Substitution in Structural Analogues of S-Adenosylmethionine (dagger). J Med Chem 52:1388-407 (2009) [PubMed] Article Target
Name:
S-adenosylmethionine decarboxylase proenzyme
Synonyms:
AMD | AMD1 | DCAM_HUMAN | S-Adenosylmethionine Decarboxylase (AdoMetDC) | S-adenosylmethionine decarboxylase 1 | SAMDC
Type:
Heterotetramer of two alpha and two beta chains
Mol. Mass.:
38337.21
Organism:
Homo sapiens (Human)
Description:
For the production of protein for the hAdoMetDC enzyme assays, the plasmid containing (H)6 tag at the carboxyl end (replacing -QQQQQS) was used.
Residue:
334
Sequence:
MEAAHFFEGTEKLLEVWFSRQQPDANQGSGDLRTIPRSEWDILLKDVQCSIISVTKTDKQEAYVLSESSMFVSKRRFILKTCGTTLLLKALVPLLKLARDYSGFDSIQSFFYSRKNFMKPSHQGYPHRNFQEEIEFLNAIFPNGAAYCMGRMNSDCWYLYTLDFPESRVISQPDQTLEILMSELDPAVMDQFYMKDGVTAKDVTRESGIRDLIPGSVIDATMFNPCGYSMNGMKSDGTYWTIHITPEPEFSYVSFETNLSQTSYDDLIRKVVEVFKPGKFVTTLFVNQSSKCRTVLASPQKIEGFKRLDCQSAMFNDYNFVFTSFAKKQQQQQS
Inhibitor
Name:
BDBM28437
Synonyms:
3-({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}(methyl)amino)propanehydrazide | AdoMet substrate analogue, 17l
Type:
Small organic molecule
Emp. Form.:
C14H22N8O4
Mol. Mass.:
366.3757
SMILES:
CN(CCC(=O)NN)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12
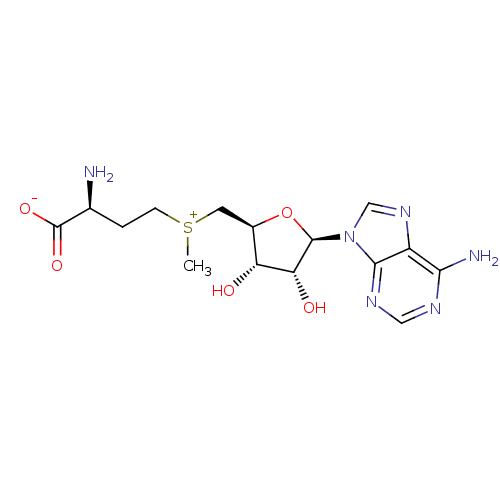
Substrate
Name:
BDBM28422
Synonyms:
(2S)-2-amino-4-({[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}(methyl)sulfanylium)butanoate | AdoMet | S-Adenosylmethionine | S-adenosyl-L-[carboxy-14C]methionine | [14COOH]AdoMet
Type:
radiolabeled substrate
Emp. Form.:
C15H22N6O5S
Mol. Mass.:
398.437
SMILES:
C[S+](CC[C@H](N)C([O-])=O)C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12