Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Trifunctional purine biosynthetic protein adenosine-3
Ligand
BDBM50022740
Substrate
n/a
Meas. Tech.
ChEMBL_69925 (CHEMBL678805)
IC50
>20000±n/a nM
Citation
 Piper, JR; McCaleb, GS; Montgomery, JA; Kisliuk, RL; Gaumont, Y; Thorndike, J; Sirotnak, FM Synthesis and antifolate activity of 5-methyl-5,10-dideaza analogues of aminopterin and folic acid and an alternative synthesis of 5,10-dideazatetrahydrofolic acid, a potent inhibitor of glycinamide ribonucleotide formyltransferase. J Med Chem 31:2164-9 (1988) [PubMed] Article
Piper, JR; McCaleb, GS; Montgomery, JA; Kisliuk, RL; Gaumont, Y; Thorndike, J; Sirotnak, FM Synthesis and antifolate activity of 5-methyl-5,10-dideaza analogues of aminopterin and folic acid and an alternative synthesis of 5,10-dideazatetrahydrofolic acid, a potent inhibitor of glycinamide ribonucleotide formyltransferase. J Med Chem 31:2164-9 (1988) [PubMed] Article More Info.:
Target
Name:
Trifunctional purine biosynthetic protein adenosine-3
Synonyms:
GAR transformylase | Gart | PUR2_MOUSE
Type:
PROTEIN
Mol. Mass.:
107504.27
Organism:
Mus musculus
Description:
ChEMBL_497843
Residue:
1010
Sequence:
MAARVLVIGSGGREHTLAWKLAQSPQVKQVLVAPGNAGTACAGKISNAAVSVNDHSALAQFCKDEKIELVVVGPEAPLAAGIVGDLTSAGVRCFGPTAQAAQLESSKKFAKEFMDRHEIPTAQWRAFTNPEDACSFITSANFPALVVKASGLAAGKGVIVAKSQAEACRAVQEIMQEKSFGAAGETVVVEEFLEGEEVSCLCFTDGKTVAEMPPAQDHKRLLDGDEGPNTGGMGAYCPAPQVSKDLLVKIKNTILQRAVDGMQQEGAPYTGILYAGIMLTKDGPKVLEFNCRFGDPECQVILPLLKSDLYEVMQSTLDGLLSASLPVWLENHSAVTVVMASKGYPGAYTKGVEITGFPEAQALGLQVFHAGTALKDGKVVTSGGRVLTVTAVQENLMSALAEARKGLAALKFEGAIYRKDIGFRAVAFLQRPRGLTYKDSGVDIAAGNMLVKKIQPLAKATSRPGCSVDLGGFAGLFDLKAAGFKDPLLASGTDGVGTKLKIAQLCNKHDSIGQDLVAMCVNDILAQGAEPLFFLDYFSCGKLDLSTTEAVIAGIAAACQQAGCALLGGETAEMPNMYPPGEYDLAGFAVGAMERHQKLPQLERITEGDAVIGVASSGLHSNGFSLVRKIVERSSLQYSSPAPGGCGDQTLGDLLLTPTRIYSHSLLPIIRSGRVKAFAHITGGGLLENIPRVLPQKFGVDLDASTWRVPKVFSWLQQEGELSEEEMARTFNCGIGAALVVSKDQAEQVLHDVRRRQEEAWVIGSVVACPEDSPRVRVKNLIETIQTNGSLVANGFLKSNFPVQQKKARVAVLISGTGSNLQALIDSTRDPKSSSHIVLVISNKAAVAGLDRAERAGIPTRVINHKLYKNRVEFDNAVDHVLEEFSVDIVCLAGFMRILSGPFVRKWDGKMLNIHPSLLPSFKGSNAHEQVLEAGVTITGCTVHFVAEDVDAGQIILQEAVPVRRGDTVATLSERVKVAEHKIFPAALQLVASGAVQLREDGKIHWAKEQ
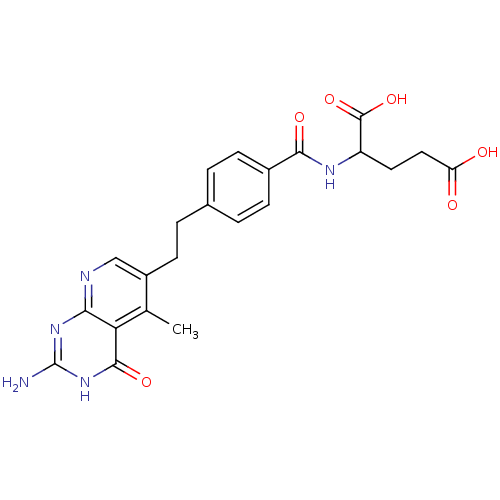
Inhibitor
Name:
BDBM50022740
Synonyms:
2-{4-[2-(2-Amino-5-methyl-4-oxo-3,4-dihydro-pyrido[2,3-d]pyrimidin-6-yl)-ethyl]-benzoylamino}-pentanedioic acid | CHEMBL66864
Type:
Small organic molecule
Emp. Form.:
C22H23N5O6
Mol. Mass.:
453.4479
SMILES:
Cc1c(CCc2ccc(cc2)C(=O)NC(CCC(O)=O)C(O)=O)cnc2nc(N)[nH]c(=O)c12