Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
72 kDa type IV collagenase
Ligand
BDBM50247539
Substrate
n/a
Meas. Tech.
ChEMBL_1778594 (CHEMBL4235586)
IC50
266±n/a nM
Citation
More Info.:
Target
Name:
72 kDa type IV collagenase
Synonyms:
72 kDa gelatinase | 72 kDa type IV collagenase precursor | CLG4A | Gelatinase A | Gelatinase A (MMP-2) | MMP2 | MMP2_HUMAN | Matrix metalloproteinase-2 | Matrix metalloproteinase-2 (MMP 2) | Matrix metalloproteinase-2 (MMP2) | Matrix metalloproteinases 2 (MMP-2) | TBE-1
Type:
Enzyme
Mol. Mass.:
73870.36
Organism:
Homo sapiens (Human)
Description:
P08253
Residue:
660
Sequence:
MEALMARGALTGPLRALCLLGCLLSHAAAAPSPIIKFPGDVAPKTDKELAVQYLNTFYGCPKESCNLFVLKDTLKKMQKFFGLPQTGDLDQNTIETMRKPRCGNPDVANYNFFPRKPKWDKNQITYRIIGYTPDLDPETVDDAFARAFQVWSDVTPLRFSRIHDGEADIMINFGRWEHGDGYPFDGKDGLLAHAFAPGTGVGGDSHFDDDELWTLGEGQVVRVKYGNADGEYCKFPFLFNGKEYNSCTDTGRSDGFLWCSTTYNFEKDGKYGFCPHEALFTMGGNAEGQPCKFPFRFQGTSYDSCTTEGRTDGYRWCGTTEDYDRDKKYGFCPETAMSTVGGNSEGAPCVFPFTFLGNKYESCTSAGRSDGKMWCATTANYDDDRKWGFCPDQGYSLFLVAAHEFGHAMGLEHSQDPGALMAPIYTYTKNFRLSQDDIKGIQELYGASPDIDLGTGPTPTLGPVTPEICKQDIVFDGIAQIRGEIFFFKDRFIWRTVTPRDKPMGPLLVATFWPELPEKIDAVYEAPQEEKAVFFAGNEYWIYSASTLERGYPKPLTSLGLPPDVQRVDAAFNWSKNKKTYIFAGDKFWRYNEVKKKMDPGFPKLIADAWNAIPDNLDAVVDLQGGGHSYFFKGAYYLKLENQSLKSVKFGSIKSDWLGC
Inhibitor
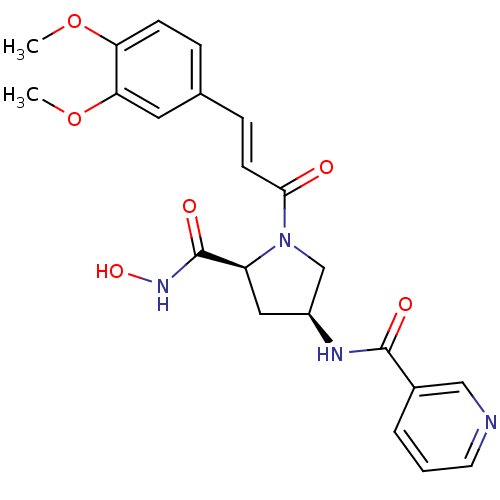
Name:
BDBM50247539
Synonyms:
CHEMBL472915 | N-((3S,5S)-1-((E)-3-(3,4-dimethoxyphenyl)acryloyl)-5-(hydroxycarbamoyl)pyrrolidin-3-yl)nicotinamide | N-((3S,5S)-1-(3-(3,4-dimethoxyphenyl)acryloyl)-5-(hydroxycarbamoyl)pyrrolidin-3-yl)nicotinamide
Type:
Small organic molecule
Emp. Form.:
C22H24N4O6
Mol. Mass.:
440.4492
SMILES:
COc1ccc(\C=C\C(=O)N2C[C@H](C[C@H]2C(=O)NO)NC(=O)c2cccnc2)cc1OC |r|