Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuraminidase
Ligand
BDBM4994
Substrate
n/a
Meas. Tech.
ChEMBL_709406 (CHEMBL1665840)
Ki
4.9±n/a nM
Citation
 Govorkova, EA; Ilyushina, NA; McClaren, JL; Naipospos, TS; Douangngeun, B; Webster, RG Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model. Antimicrob Agents Chemother 53:3088-96 (2009) [PubMed] Article
Govorkova, EA; Ilyushina, NA; McClaren, JL; Naipospos, TS; Douangngeun, B; Webster, RG Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model. Antimicrob Agents Chemother 53:3088-96 (2009) [PubMed] Article More Info.:
Target
Name:
Neuraminidase
Synonyms:
n/a
Type:
PROTEIN
Mol. Mass.:
49064.69
Organism:
Influenza A virus
Description:
ChEMBL_709401
Residue:
449
Sequence:
MNPNQKIITIGSICMVIGMVSLMLQIGNMISIWLSHSIQTGNQHQAESISNNNLLTENAVASVTLAGNSSLCPIRGWAVHSKDNSIRIGSKGDVFVIREPFISCSHLECRTFFLTQGALLNDKHSNGTVKDRSPHRTLMSCPVGEAPSPYNSRFESVAWSASACHDGISWLTIGISGPDNGAVAVLKYNGIITDTIKSWRNNILRTQESECACVNGSCFTVMTDGPSNGQASYKIFKMEKGKVVKSVELDAPNYHYEECSCYPDAGEITCVCRDNWHGSNRPWVSFNQNLEYQIGYICSGVFGDNPRPNDGTGSCGPMSPNGAYGVKGFSFKYGNGVWIGRTKSTNSRSGFEMIWDPNGWTGTDSSFSVKQDIVAITDWSGYSGSFVQHPELTGLDCIRPCFWVELIRGRPKESTIWTSGSSISFCGVNGDTVSWSWPDGAELPFIIDK
Inhibitor
Name:
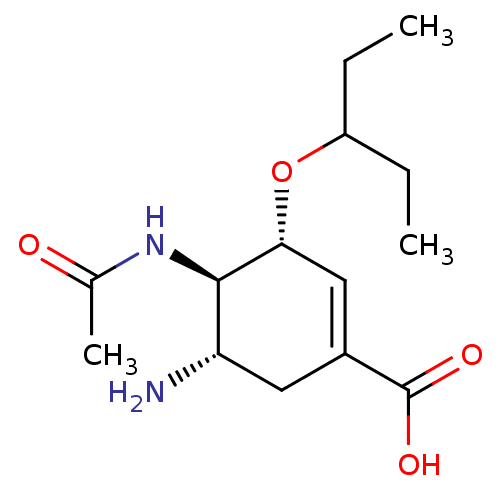
BDBM4994
Synonyms:
(3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)cyclohex-1-ene-1-carboxylic acid | CHEMBL674 | GS4071 | Oseltamivir carboxylate
Type:
Small organic molecule
Emp. Form.:
C14H24N2O4
Mol. Mass.:
284.3514
SMILES:
CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7|