Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50356597
Substrate
n/a
Meas. Tech.
ChEMBL_776115 (CHEMBL1912811)
IC50
1400±n/a nM
Citation
 Wang, W; Devasthale, P; Wang, A; Harrity, T; Egan, D; Morgan, N; Cap, M; Fura, A; Klei, HE; Kish, K; Weigelt, C; Sun, L; Levesque, P; Li, YX; Zahler, R; Kirby, MS; Hamann, LG 7-Oxopyrrolopyridine-derived DPP4 inhibitors-mitigation of CYP and hERG liabilities via introduction of polar functionalities in the active site. Bioorg Med Chem Lett 21:6646-51 (2011) [PubMed] Article
Wang, W; Devasthale, P; Wang, A; Harrity, T; Egan, D; Morgan, N; Cap, M; Fura, A; Klei, HE; Kish, K; Weigelt, C; Sun, L; Levesque, P; Li, YX; Zahler, R; Kirby, MS; Hamann, LG 7-Oxopyrrolopyridine-derived DPP4 inhibitors-mitigation of CYP and hERG liabilities via introduction of polar functionalities in the active site. Bioorg Med Chem Lett 21:6646-51 (2011) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
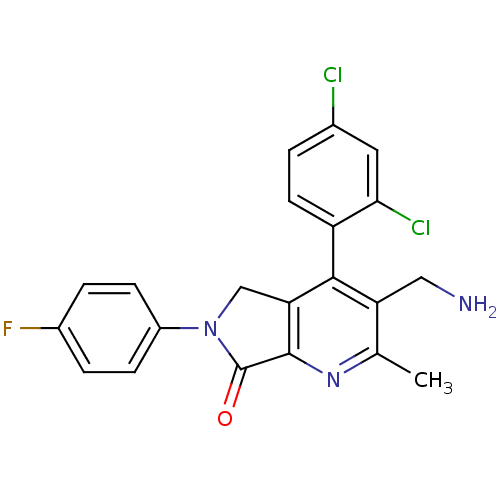
Inhibitor
Name:
BDBM50356597
Synonyms:
CHEMBL1910107
Type:
Small organic molecule
Emp. Form.:
C21H16Cl2FN3O
Mol. Mass.:
416.276
SMILES:
Cc1nc2C(=O)N(Cc2c(c1CN)-c1ccc(Cl)cc1Cl)c1ccc(F)cc1 |(54.92,-34.84,;53.58,-35.61,;52.25,-34.85,;50.92,-35.62,;49.46,-35.15,;48.98,-33.68,;48.55,-36.39,;49.45,-37.64,;50.92,-37.16,;52.25,-37.93,;53.59,-37.16,;54.93,-37.93,;56.26,-37.16,;52.25,-39.47,;50.92,-40.24,;50.92,-41.78,;52.25,-42.55,;52.25,-44.09,;53.59,-41.77,;53.59,-40.24,;54.92,-39.46,;47.02,-36.39,;46.25,-35.05,;44.71,-35.05,;43.94,-36.39,;42.4,-36.39,;44.71,-37.72,;46.25,-37.72,)|