Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Nuclear receptor subfamily 1 group I member 3
Ligand
BDBM50187243
Substrate
n/a
Meas. Tech.
ChEMBL_1555045 (CHEMBL3767623)
IC50
3000±n/a nM
Citation
More Info.:
Target
Name:
Nuclear receptor subfamily 1 group I member 3
Synonyms:
CAR | NR1I3 | NR1I3_HUMAN
Type:
PROTEIN
Mol. Mass.:
39953.17
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1434936
Residue:
352
Sequence:
MASREDELRNCVVCGDQATGYHFNALTCEGCKGFFRRTVSKSIGPTCPFAGSCEVSKTQRRHCPACRLQKCLDAGMRKDMILSAEALALRRAKQAQRRAQQTPVQLSKEQEELIRTLLGAHTRHMGTMFEQFVQFRPPAHLFIHHQPLPTLAPVLPLVTHFADINTFMVLQVIKFTKDLPVFRSLPIEDQISLLKGAAVEICHIVLNTTFCLQTQNFLCGPLRYTIEDGARVSPTVGFQVEFLELLFHFHGTLRKLQLQEPEYVLLAAMALFSPDRPGVTQRDEIDQLQEEMALTLQSYIKGQQRRPRDRFLYAKLLGLLAELRSINEAYGYQIQHIQGLSAMMPLLQEICS
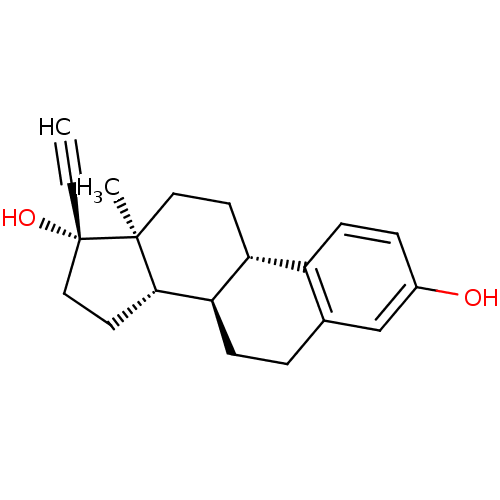
Inhibitor
Name:
BDBM50187243
Synonyms:
17-ethinyl-3,17-estradiol | 17-ethinyl-3,17-oestradiol | 17-ethinylestradiol | 17alpha-Ethinyl estradiol | 17alpha-ethynylestra-1,3,5(10)-triene-3,17beta-diol | 17alpha-ethynylestradiol | CHEMBL691 | ETHINYL ESTRADIOL | Ethinylestradiol | Ethynyl estradiol | ethinyloestradiol
Type:
Small organic molecule
Emp. Form.:
C20H24O2
Mol. Mass.:
296.4034
SMILES:
C[C@]12CC[C@H]3[C@@H](CCc4cc(O)ccc34)[C@@H]1CC[C@@]2(O)C#C |r|