Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mineralocorticoid receptor
Ligand
BDBM18207
Substrate
n/a
Meas. Tech.
ChEBML_1625702
IC50
40±n/a nM
Citation
 Hemmerling, M; Edman, K; Lepistö, M; Eriksson, A; Ivanova, S; Dahmén, J; Rehwinkel, H; Berger, M; Hendrickx, R; Dearman, M; Jensen, TJ; Wissler, L; Hansson, T Discovery of indazole ethers as novel, potent, non-steroidal glucocorticoid receptor modulators. Bioorg Med Chem Lett 26:5741-5748 (2016) [PubMed] Article
Hemmerling, M; Edman, K; Lepistö, M; Eriksson, A; Ivanova, S; Dahmén, J; Rehwinkel, H; Berger, M; Hendrickx, R; Dearman, M; Jensen, TJ; Wissler, L; Hansson, T Discovery of indazole ethers as novel, potent, non-steroidal glucocorticoid receptor modulators. Bioorg Med Chem Lett 26:5741-5748 (2016) [PubMed] Article More Info.:
Target
Name:
Mineralocorticoid receptor
Synonyms:
MCR | MCR_HUMAN | MLR | MR | NR3C2 | Nuclear receptor subfamily 3 group C member 2
Type:
Enzyme
Mol. Mass.:
107076.42
Organism:
Homo sapiens (Human)
Description:
P08235
Residue:
984
Sequence:
METKGYHSLPEGLDMERRWGQVSQAVERSSLGPTERTDENNYMEIVNVSCVSGAIPNNSTQGSSKEKQELLPCLQQDNNRPGILTSDIKTELESKELSATVAESMGLYMDSVRDADYSYEQQNQQGSMSPAKIYQNVEQLVKFYKGNGHRPSTLSCVNTPLRSFMSDSGSSVNGGVMRAVVKSPIMCHEKSPSVCSPLNMTSSVCSPAGINSVSSTTASFGSFPVHSPITQGTPLTCSPNVENRGSRSHSPAHASNVGSPLSSPLSSMKSSISSPPSHCSVKSPVSSPNNVTLRSSVSSPANINNSRCSVSSPSNTNNRSTLSSPAASTVGSICSPVNNAFSYTASGTSAGSSTLRDVVPSPDTQEKGAQEVPFPKTEEVESAISNGVTGQLNIVQYIKPEPDGAFSSSCLGGNSKINSDSSFSVPIKQESTKHSCSGTSFKGNPTVNPFPFMDGSYFSFMDDKDYYSLSGILGPPVPGFDGNCEGSGFPVGIKQEPDDGSYYPEASIPSSAIVGVNSGGQSFHYRIGAQGTISLSRSARDQSFQHLSSFPPVNTLVESWKSHGDLSSRRSDGYPVLEYIPENVSSSTLRSVSTGSSRPSKICLVCGDEASGCHYGVVTCGSCKVFFKRAVEGQHNYLCAGRNDCIIDKIRRKNCPACRLQKCLQAGMNLGARKSKKLGKLKGIHEEQPQQQQPPPPPPPPQSPEEGTTYIAPAKEPSVNTALVPQLSTISRALTPSPVMVLENIEPEIVYAGYDSSKPDTAENLLSTLNRLAGKQMIQVVKWAKVLPGFKNLPLEDQITLIQYSWMCLSSFALSWRSYKHTNSQFLYFAPDLVFNEEKMHQSAMYELCQGMHQISLQFVRLQLTFEEYTIMKVLLLLSTIPKDGLKSQAAFEEMRTNYIKELRKMVTKCPNNSGQSWQRFYQLTKLLDSMHDLVSDLLEFCFYTFRESHALKVEFPAMLVEIISDQLPKVESGNAKPLYFHRK
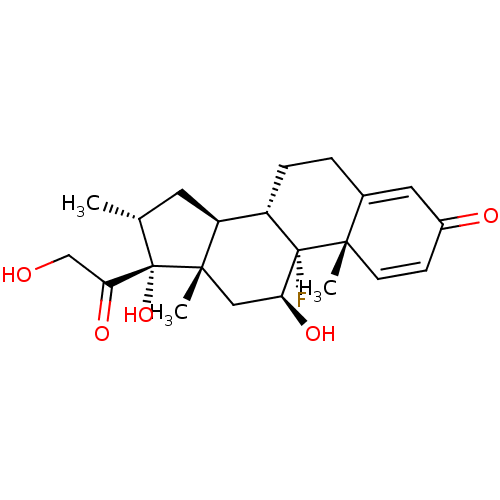
Inhibitor
Name:
BDBM18207
Synonyms:
(1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,13,15-trimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one | US10869929, Compound Dexamethasone | US11554172, Compound Dexamethasone | dexamethasone | dexamethasone (tetramethyl-rhodamine conjugated )
Type:
Steroid
Emp. Form.:
C22H29FO5
Mol. Mass.:
392.4611
SMILES:
[H][C@@]12C[C@@H](C)[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1(F)[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |c:28,t:24|