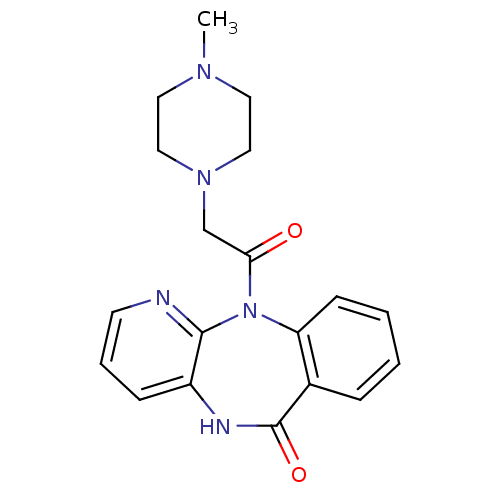
BDBM39341 11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6H-pyrido[2,3-b][1,4]benzodiazepin-6-one::11-[2-(4-methyl-1-piperazinyl)-1-oxoethyl]-5H-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride::11-[2-(4-methylpiperazin-1-yl)acetyl]-5H-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride::11-[2-(4-methylpiperazin-1-yl)ethanoyl]-5H-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride::11-[2-(4-methylpiperazino)acetyl]-5H-pyrido[2,3-b][1,4]benzodiazepin-6-one;hydrochloride::CHEMBL9967::MLS000069702::PIRENZEPINE::PIRENZEPINE DIHYDROCHLORIDE::SMR000058502::cid_185248
SMILES CN1CCN(CC(=O)N2c3ccccc3C(=O)Nc3cccnc23)CC1
InChI Key InChIKey=RMHMFHUVIITRHF-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 39341
Found 3 hits for monomerid = 39341
Affinity DataKi: 3.60nMAssay Description:Binding affinity against Muscarinic acetylcholine receptor M1 by displacement of [3H]pirenzepine in bovine striatumMore data for this Ligand-Target Pair
Affinity DataKi: 377nMAssay Description:Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heartMore data for this Ligand-Target Pair
Affinity DataKi: 1.83E+4nMAssay Description:Evaluated for the inhibition of adenylate cyclase at M2 receptor in rat heartMore data for this Ligand-Target Pair