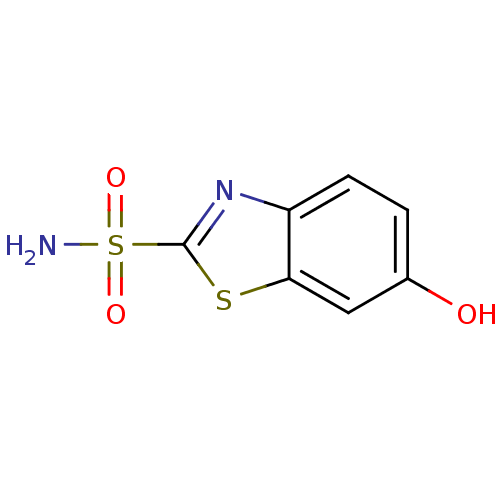
BDBM10874 6-hydroxy-1,3-benzothiazole-2-sulfonamide::CHEMBL6685::aromatic/heteroaromatic sulfonamide 19
SMILES NS(=O)(=O)c1nc2ccc(O)cc2s1
InChI Key InChIKey=NOOBQTYVTDBXTL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 10874
Found 4 hits for monomerid = 10874
Affinity DataKi: 30nMAssay Description:Inhibition of recombinant human carbonic anhydrase-2 by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase, alpha family(Thiomicrospira crunogena (strain XCL-2))
Universita Degli Studi Di Firenze
Curated by ChEMBL
Universita Degli Studi Di Firenze
Curated by ChEMBL
Affinity DataKi: 41nMAssay Description:Inhibition of recombinant Thiomicrospira crunogena XCL-2 carbonic anhydrase by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
TargetCarbonic anhydrase(Sulfurihydrogenibium sp. (strain YO3AOP1))
Universita Degli Studi Di Firenze
Curated by ChEMBL
Universita Degli Studi Di Firenze
Curated by ChEMBL
Affinity DataKi: 66nMAssay Description:Inhibition of recombinant Sulfurihydrogenibium yellowstonense YO3AOP1 carbonic anhydrase by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair
Affinity DataKi: 95nMAssay Description:Inhibition of recombinant human carbonic anhydrase-1 by stopped flow CO2 hydrase assayMore data for this Ligand-Target Pair