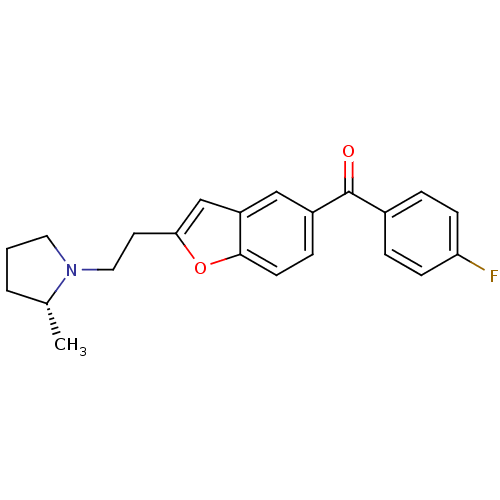
BDBM50158610 (4-Fluoro-phenyl)-{2-[2-((R)-2-methyl-pyrrolidin-1-yl)-ethyl]-benzofuran-5-yl}-methanone::CHEMBL555146
SMILES C[C@@H]1CCCN1CCc1cc2cc(ccc2o1)C(=O)c1ccc(F)cc1
InChI Key InChIKey=VGSYEFOBFMUWGO-OAHLLOKOSA-N
Data 40 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50158610
Found 3 hits for monomerid = 50158610
Affinity DataKi: 0.270nMAssay Description:In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing human histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:In vitro binding affinity was determined as displacement of [3H]N-R-methylhistamine from C6 cell membranes expressing rat histamine H3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.90nMAssay Description:Binding affinity to human H3 receptorMore data for this Ligand-Target Pair