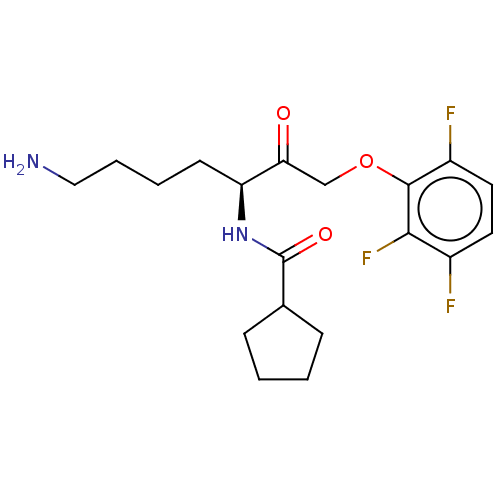
BDBM453275 BDBM453327::US10730826, Compound 1a-non-racemic::US10730826, Compound 1a-racemic::US11325884, Compound 1
SMILES NCCCC[C@H](NC(=O)C1CCCC1)C(=O)COc1c(F)ccc(F)c1F
InChI Key InChIKey=OLIMBXKACVCIMM-HNNXBMFYSA-N
Data 25 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 25 hits for monomerid = 453275
Found 25 hits for monomerid = 453275
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 2.95E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 4.68E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 117nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: 4.68E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair
Affinity DataIC50: <0.0500nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain (Kgp) were measured in a fluorogenic assay similar t...More data for this Ligand-Target Pair
Affinity DataIC50: 117nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 4.68E+3nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of cathepsins B, H, K, L, and S were measured in similar assays. Boc-Leu...More data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:The capacities of compounds of the present invention to inhibit the activity of lysine gingipain were measured in a fluorogenic assay similar to thos...More data for this Ligand-Target Pair