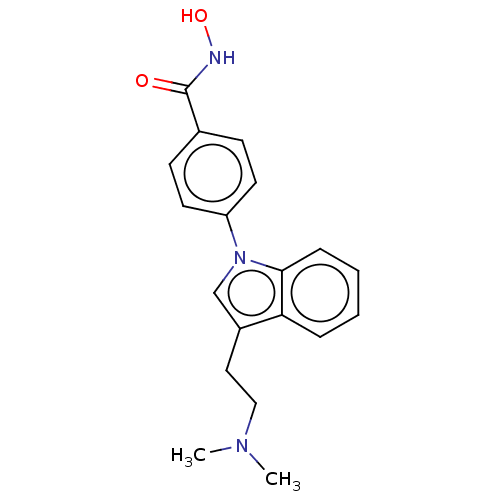
BDBM218229 US9249087, Table 1 , Compound 6
SMILES CN(C)CCc1cn(-c2ccc(cc2)C(=O)NO)c2ccccc12
InChI Key InChIKey=PAVSNNNECFFYLN-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 218229
Found 3 hits for monomerid = 218229
Affinity DataIC50: 12.5nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 422nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair
Affinity DataIC50: 5.86E+3nMpH: 8.0Assay Description:HDAC assay is performed using fluorescently-labeled acetylated substrate, which comprises an acetylated lysine side chain. After incubation with HDAC...More data for this Ligand-Target Pair