BDBM256459 US10329256, Example 3::US9487512, 3::US9944601, Example 3
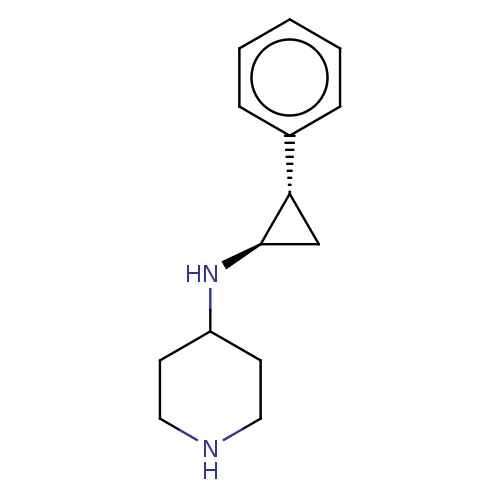
SMILES C1[C@@H](NC2CCNCC2)[C@@H]1c1ccccc1
InChI Key InChIKey=BASFYRLYJAZPPL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 38 hits for monomerid = 256459
Found 38 hits for monomerid = 256459
Affinity DataIC50: 16nMAssay Description:Inhibition of LSD1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) preincubated for 10 mins in presence of H3K4Me2 measured after 30 mins by measuring fluorescence inte...More data for this Ligand-Target Pair
Affinity DataIC50: 24nMAssay Description:Inhibition of recombinant LSD1 (unknown origin) (catalytic domain 157 to 852 residues) expressed in Escherichia coli BL21 using H3K4me1 as substrate ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Human)
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataIC50: 42nMpH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:Briefly, a fixed amount of LSD1 was incubated on ice for 15 minutes, in the absence and/or in the presence of at least eight 3-fold serial dilutions ...More data for this Ligand-Target Pair
Affinity DataIC50: 42nMAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
TargetGlutathione S-transferase P/Isoform 2 of Lysine-specific histone demethylase 1A (2) [158-876](Human)
Oryzon Genomics
US Patent
Oryzon Genomics
US Patent
Affinity DataKi: 50nM ΔG°: -10.4kcal/molepH: 7.4 T: 2°CAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:Briefly, a fixed amount of LSD1 was incubated on ice for 15 minutes, in the absence and/or in the presence of at least eight 3-fold serial dilutions ...More data for this Ligand-Target Pair
Affinity DataKi: 55nMAssay Description:The compounds of the invention can be tested for their ability to inhibit LSD1. The ability of the compounds of the invention to inhibit LSD1 can be ...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Binding affinity to LSD1 (unknown origin) using H3K4me2 as substrate assessed as apparent inhibition constant by HRP-coupled amplex red assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of SERT (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of NET (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of KCNQ1/minK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of Kv1.5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of PXR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of OATP1B1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of LCK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMAssay Description:Inhibition of Aurora B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of MAO-B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PDE4B (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of AChE (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of PDE3A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Nav1.5 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-5.67kcal/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-5.67kcal/molepH: 7.5 T: 2°CAssay Description:Human recombinant monoamine oxidase proteins MAO-A and MAO-B were purchased from Sigma Aldrich. MAOs catalyze the oxidative deamination of primary, s...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+5nMAssay Description:Activation of AhR (unknown origin)More data for this Ligand-Target Pair
Affinity DataKd: 4.25E+5nMAssay Description:Binding affinity to LSD1 (unknown origin) assessed as dissociation constant by SPR assayMore data for this Ligand-Target Pair