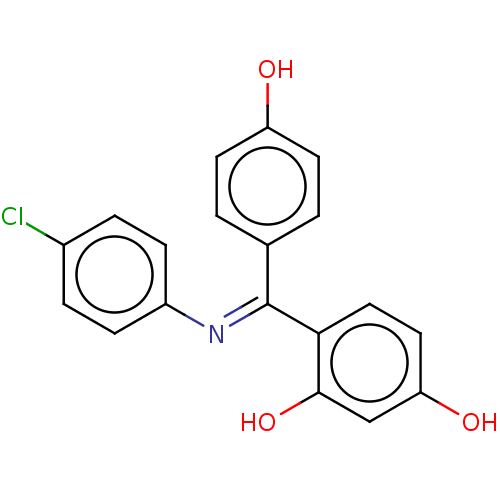
BDBM50005605 CHEMBL3234632
SMILES Oc1ccc(cc1)C(=N/c1ccc(Cl)cc1)\c1ccc(O)cc1O
InChI Key InChIKey=JADLZJQKHPGJHN-XUTLUUPISA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50005605
Found 3 hits for monomerid = 50005605
TargetEstrogen receptor beta(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Antagonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as inhibition of 17beta-estradiol-induced transcriptional acti...More data for this Ligand-Target Pair
TargetEstrogen receptor beta(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataEC50: 385nMAssay Description:Agonist activity at ERbeta (unknown origin) expressed in human HepG2 cells assessed as transcriptional activation after 24 hrs by ERE-luciferase repo...More data for this Ligand-Target Pair
TargetEstrogen receptor(Human)
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Key Laboratory of Combinatorial Biosynthesis and Drug Discovery (Wuhan University)
Curated by ChEMBL
Affinity DataEC50: 4nMAssay Description:Agonist activity at ERalpha (unknown origin) expressed in human HepG2 cells assessed as transcriptional activation after 24 hrs by ERE-luciferase rep...More data for this Ligand-Target Pair