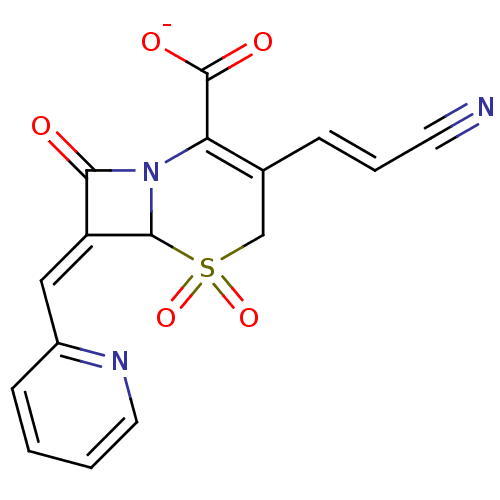
BDBM50088827 CHEMBL355558::Sodium; 3-((E)-2-cyano-vinyl)-5,5,8-trioxo-7-[1-pyridin-2-yl-meth-(Z)-ylidene]-5lambda*6*-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
SMILES [O-]C(=O)C1=C(CS(=O)(=O)C2N1C(=O)\C2=C\c1ccccn1)\C=C\C#N
InChI Key InChIKey=ITJYYTDFHUHVOA-UHFFFAOYSA-M
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50088827
Found 4 hits for monomerid = 50088827
Affinity DataIC50: 10nMAssay Description:The compound was evaluated for inhibition against Class C beta-lactamase derived from Enterobacter cloacae P99More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The compound was evaluated for inhibition against the GC1 extended spectrum Class C beta-lactamaseMore data for this Ligand-Target Pair
TargetBeta-lactamase(Pseudomonas aeruginosa (g-Proteobacteria))
Southern Methodist University
Curated by ChEMBL
Southern Methodist University
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of class A TEM-1 beta-lactamase derived from Enterobacter cloacaeMore data for this Ligand-Target Pair
Affinity DataIC50: 720nMAssay Description:Inhibition of class A beta-lactamase derived from Staphylococcus aureus strain PC1More data for this Ligand-Target Pair