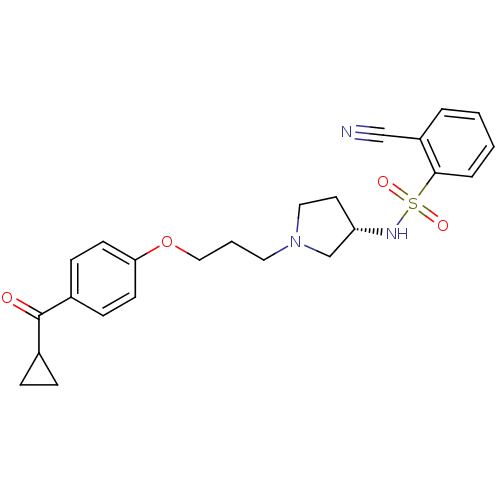
BDBM50119708 2-Cyano-N-{(S)-1-[3-(4-cyclopropanecarbonyl-phenoxy)-propyl]-pyrrolidin-3-yl}-benzenesulfonamide::CHEMBL420108
SMILES O=C(C1CC1)c1ccc(OCCCN2CC[C@@H](C2)NS(=O)(=O)c2ccccc2C#N)cc1
InChI Key InChIKey=QFTNRJFCJNHEPW-UHFFFAOYSA-N
Data 3 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50119708
Found 3 hits for monomerid = 50119708
Affinity DataKi: 11nMAssay Description:Binding affinity towards rats Histamine type 3 (H3) receptorMore data for this Ligand-Target Pair
Affinity DataKi: 710nMAssay Description:Binding affinity towards human Histamine H2 receptor (For compound 11)More data for this Ligand-Target Pair
Affinity DataKi: 6.20E+3nMAssay Description:Binding affinity to the human Histamine H1 receptorMore data for this Ligand-Target Pair