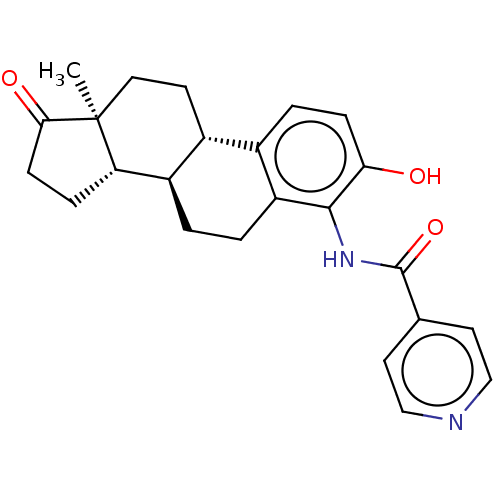
BDBM50123029 CHEMBL3623221
SMILES [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])c3ccc(O)c(NC(=O)c4ccncc4)c3CC[C@@]21[H]
InChI Key InChIKey=GNRLSMLEQLVWTH-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50123029
Found 2 hits for monomerid = 50123029
Affinity DataKi: 1.53E+3nMAssay Description:Competitive inhibition of human aromatase extracted from placental microsomes after 5 mins by Dixon plot analysis in presence of [1beta-3H]ADMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+4nMAssay Description:Inhibition of human placental aromatase assessed as conversion of 3H2O from [1beta-3H]AD after 20 mins by Siiteri and Thompson methodMore data for this Ligand-Target Pair