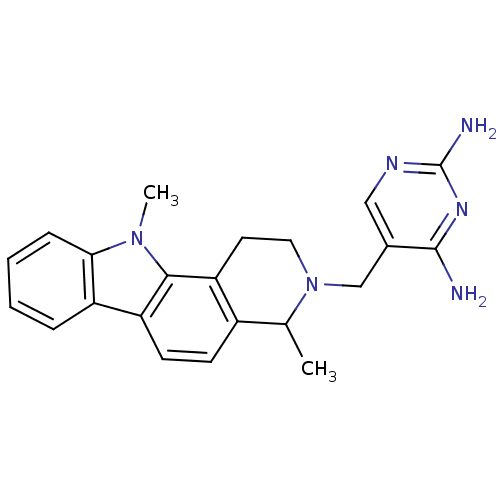
BDBM50128534 5-(4,11-Dimethyl-1,2,4,11-tetrahydro-pyrido[4,3-a]carbazol-3-ylmethyl)-pyrimidine-2,4-diamine::CHEMBL308758
SMILES CC1N(Cc2cnc(N)nc2N)CCc2c1ccc1c3ccccc3n(C)c21
InChI Key InChIKey=KZNVWZDVHNNDIM-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50128534
Found 4 hits for monomerid = 50128534
Affinity DataIC50: 4nMAssay Description:Antibacterial activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Inhibitory activity of TMP-susceptible Dihydrofolate reductase against Staphylococcus pneumoniae ATCC 49619More data for this Ligand-Target Pair
Affinity DataIC50: 85nMAssay Description:Antibacterial activity against TMP-Resistance Dihydrofolate reductase from Staphylococcus pneumoniae 1/1More data for this Ligand-Target Pair
TargetDihydrofolate reductase(Staphylococcus aureus (strain MW2))
F. Hoffmann-La Roche
Curated by ChEMBL
F. Hoffmann-La Roche
Curated by ChEMBL
Affinity DataIC50: 6.80E+3nMAssay Description:Inhibitory activity against TMP-Resistance Dihydrofolate reductase from Staphylococcus aureus 157/4696More data for this Ligand-Target Pair