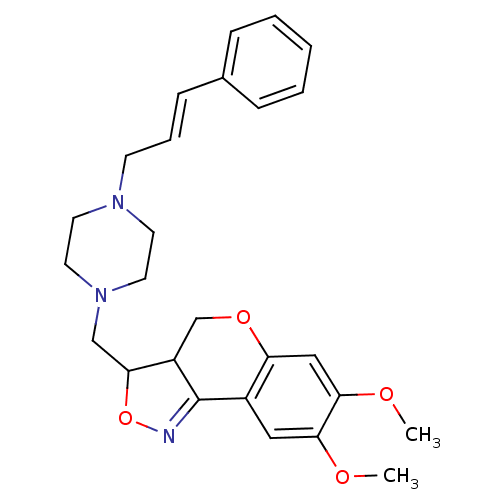
BDBM50131355 7,8-Dimethoxy-3-[4-((E)-3-phenyl-allyl)-piperazin-1-ylmethyl]-3a,4-dihydro-3H-chromeno[4,3-c]isoxazole::CHEMBL93489
SMILES COc1cc2OCC3C(CN4CCN(C\C=C\c5ccccc5)CC4)ON=C3c2cc1OC
InChI Key InChIKey=WZOMJNATDSYQHS-RMKNXTFCSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50131355
Found 4 hits for monomerid = 50131355
TargetAlpha-2C adrenergic receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 6.20nMAssay Description:In vitro binding affinity towards human adrenergic alpha-2C adrenergic receptor using [3H]-rauwolscineMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rat)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8.30nMAssay Description:In vitro binding affinity towards rat serotonin transporterMore data for this Ligand-Target Pair
TargetAlpha-2A adrenergic receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 8.80nMAssay Description:In vitro binding affinity towards human alpha-2A adrenergic receptor using [3H]-rauwolscineMore data for this Ligand-Target Pair
TargetAlpha-2B adrenergic receptor(Human)
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research & Development
Curated by ChEMBL
Affinity DataKi: 42nMAssay Description:In vitro binding affinity towards human alpha-2B adrenergic receptor using [3H]rauwolscineMore data for this Ligand-Target Pair