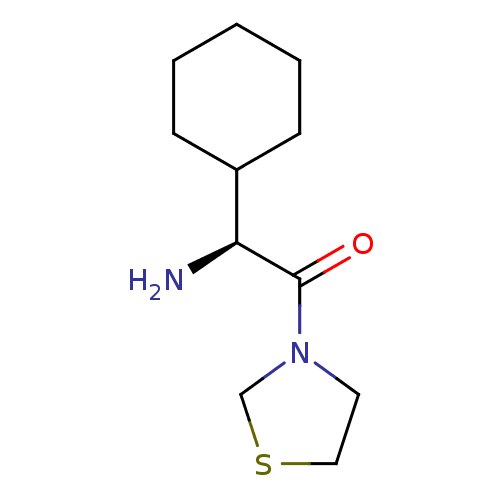
BDBM50140536 (S)-2-Amino-2-cyclohexyl-1-thiazolidin-3-yl-ethanone::CHEMBL23223
SMILES N[C@@H](C1CCCCC1)C(=O)N1CCSC1
InChI Key InChIKey=NEBWHDJOHHYIFW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50140536
Found 3 hits for monomerid = 50140536
Affinity DataIC50: 89nMAssay Description:Inhibitory activity against human Dipeptidylpeptidase IVMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibitory activity against human quiescent cell proline dipeptidase (QPP) enzymeMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.80E+4nMAssay Description:Binding affinity towards human ERG potassium ion channel was determinedMore data for this Ligand-Target Pair