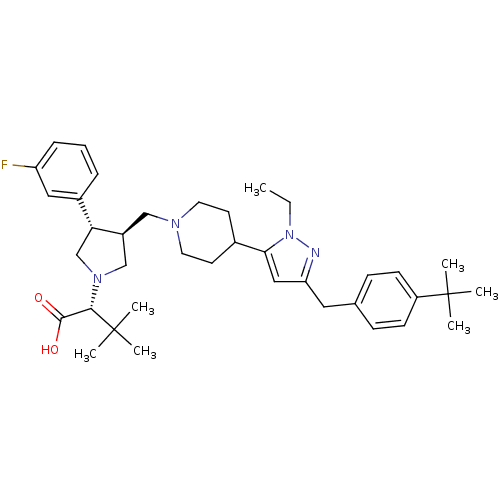
BDBM50141908 (R)-2-[(2S,3S)-3-{4-[5-(4-tert-Butyl-benzyl)-2-ethyl-2H-pyrazol-3-yl]-piperidin-1-ylmethyl}-4-(3-fluoro-phenyl)-pyrrolidin-1-yl]-3,3-dimethyl-butyric acid::CHEMBL174214
SMILES CCn1nc(Cc2ccc(cc2)C(C)(C)C)cc1C1CCN(C[C@H]2CN(C[C@@H]2c2cccc(F)c2)[C@@H](C(O)=O)C(C)(C)C)CC1
InChI Key InChIKey=JQPRDWQRAAFLTI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50141908
Found 2 hits for monomerid = 50141908
Affinity DataIC50: 0.400nMAssay Description:Inhibitory concentration to displace [125I]-MIP-1 alpha from human CX3C chemokine receptor 5 expressed in CHO cellsMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataKi: 2.10E+3nMAssay Description:Binding affinity against hERG Voltage-gated potassium channel subunit Kv11.1More data for this Ligand-Target Pair