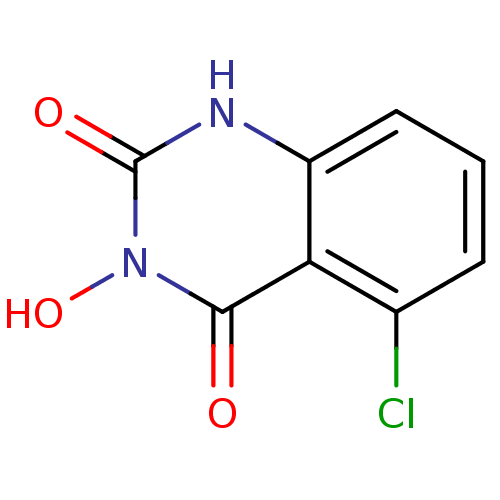
BDBM50157853 5-Chloro-3-hydroxy-4a,8a-dihydro-1H-quinazoline-2,4-dione::CHEMBL182687::US11253521, No. 11
SMILES On1c(=O)[nH]c2cccc(Cl)c2c1=O
InChI Key InChIKey=WOBAHOOLQNKVID-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50157853
Found 3 hits for monomerid = 50157853
Affinity DataIC50: 0.0140nMAssay Description:Inhibitory concentration against the Flap endonuclease-1More data for this Ligand-Target Pair
Affinity DataIC50: 0.185nMAssay Description:Inhibitory concentration against the xeroderma pigmentosum GMore data for this Ligand-Target Pair
Affinity DataIC50: 20.8nMAssay Description:Fluorogenic biochemical assays used recombinant full-length FEN-1 or catalytic domains of Exo1, XPG or GEN1 and 200 nM of a DNA substrate (formed by ...More data for this Ligand-Target Pair