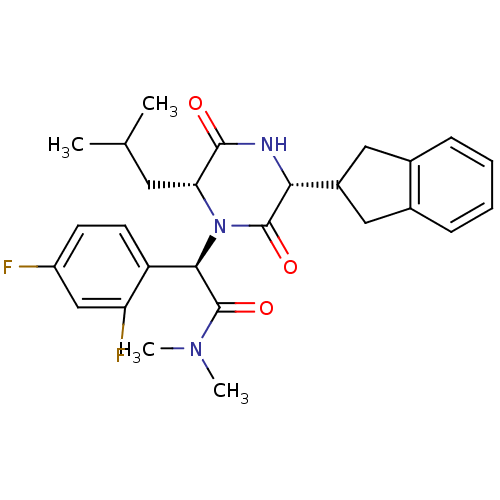
BDBM50190528 (2R)-2-(2,4-difluorophenyl)-2-[(3R,6R)-3-(2,3-dihydro-1H-inden-2-yl)-6-(2-methylpropyl)-2,5-dioxo-1-piperazinyl]-N,N-dimethylethanamide::CHEMBL377414
SMILES CC(C)C[C@H]1N([C@@H](C(=O)N(C)C)c2ccc(F)cc2F)C(=O)[C@H](NC1=O)C1Cc2ccccc2C1
InChI Key InChIKey=KUYYTTWJIWYFTJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50190528
Found 17 hits for monomerid = 50190528
Affinity DataKi: 0.0794nMAssay Description:Displacement of [3H]oxytocin from human oxytocin receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Agonist activity at human OTR expressed in CHO cells assessed as inhibition of oxytocin-induced calcium mobilization by FLIPR assayMore data for this Ligand-Target Pair
Affinity DataKi: 39.8nMAssay Description:Displacement of [3H]vasopressin from human vasopressin V2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 631nMAssay Description:Displacement of [3H]vasopressin from human vasopressin V1a receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of human CYP3A4 using diethoxyfluorescein as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human CYP2C9More data for this Ligand-Target Pair
Affinity DataKi: >1.26E+4nMAssay Description:Displacement of [3H]vasopressin from human vasopressin V1b receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+4nMAssay Description:Inhibition of human CYP3A4 using 7-benzyloxyquinoline as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:Inhibition of human CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: 4.90E+4nMAssay Description:Inhibition of CYP450 2C9 transfected in human microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+4nMAssay Description:Inhibition of CYP450 3A4 transfected in human microsome using PPR fluorogenic substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Human)
Cardiovascular and Urogenital Centre of Excellence For Drug Discovery
Curated by ChEMBL
Cardiovascular and Urogenital Centre of Excellence For Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 6.40E+4nMAssay Description:Inhibition of CYP450 1A2 transfected in human microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+4nMAssay Description:Inhibition of human CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: 8.90E+4nMAssay Description:Inhibition of CYP450 3A4 transfected in human microsome using DEF fluorogenic substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP450 2D6 transfected in human microsome using DEF fluorogenic substrateMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Human)
Cardiovascular and Urogenital Centre of Excellence For Drug Discovery
Curated by ChEMBL
Cardiovascular and Urogenital Centre of Excellence For Drug Discovery
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human CYP1A2More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP450 2C19 transfected in human microsomeMore data for this Ligand-Target Pair