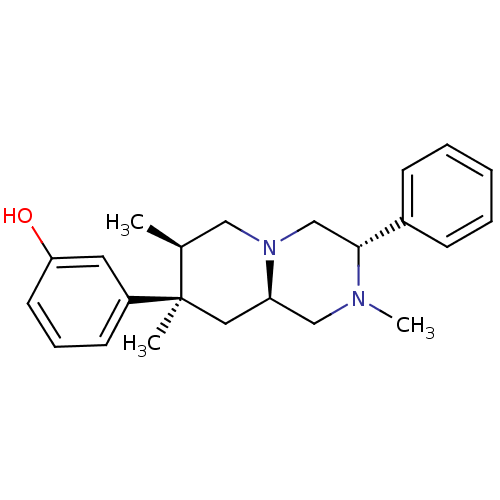
BDBM50199912 3-((3S,7R,8R,9alphaR)-2,7,8-trimethyl-3-phenyl-octahydro-1Hpyrido[1,2-R]pyrazin-8-yl)phenol::CHEMBL218520::US8580788, 64
SMILES C[C@H]1CN2C[C@@H](N(C)C[C@H]2C[C@@]1(C)c1cccc(O)c1)c1ccccc1
InChI Key InChIKey=KFASSAPEPBYWOW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50199912
Found 9 hits for monomerid = 50199912
Affinity DataIC50: 2.40nMAssay Description:Antagonist activity assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding to human kappa opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nMAssay Description:Displacement of [3H]diprenorphine from human cloned mu opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.30nM ΔG°: -11.6kcal/mole IC50: 26nMpH: 7.8 T: 2°CAssay Description:The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). More data for this Ligand-Target Pair
Affinity DataKi: 12nM ΔG°: -10.8kcal/molepH: 7.8 T: 2°CAssay Description:The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Antagonist activity assessed as inhibition of U50488-stimulated [35S]GTP-gamma-S binding to human kappa opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Antagonist activity assessed as inhibition of loperamide-stimulated [35S]GTPgammaS binding to human mu opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 580nMAssay Description:Antagonist activity assessed as inhibition of BW373U86-stimulated [35S]GTP-gamma-S binding to human delta opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 716nM ΔG°: -8.38kcal/molepH: 7.8 T: 2°CAssay Description:The receptor binding method (DeHaven and DeHaven-Hudkins, 1998) was a modification of the method of Raynor et al. (1994). More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Displacement of [3H]diprenorphine from human cloned delta opioid receptor expressed in CHO cellsMore data for this Ligand-Target Pair