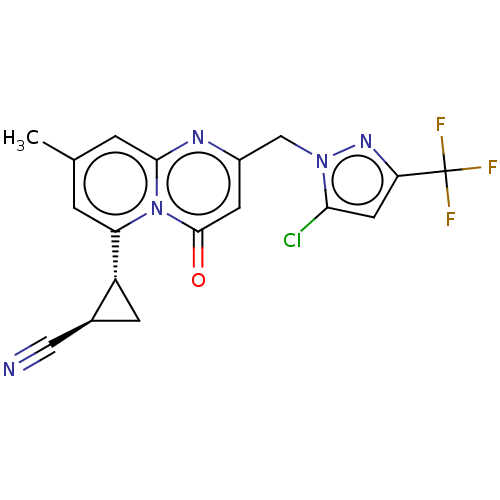
BDBM50205852 CHEMBL3976578::US10280165, Example 38
SMILES Cc1cc([C@@H]2C[C@H]2C#N)n2c(c1)nc(Cn1nc(cc1Cl)C(F)(F)F)cc2=O
InChI Key InChIKey=XSISCWOKUQLCCR-UHFFFAOYSA-N
Data 4 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50205852
Found 4 hits for monomerid = 50205852
Affinity DataEC50: 39nMAssay Description:Positive allosteric modulation of GluN1/GluN2A receptor (unknown origin) expressed in CHO cells assessed as increase in glutamate-induced calcium flu...More data for this Ligand-Target Pair
Affinity DataEC50: 210nMAssay Description:Positive allosteric modulation of human GluA2 receptor flop isoform assessed as increase in glutamate-induced calcium flux measured at time interval ...More data for this Ligand-Target Pair
Affinity DataEC50: 210nMAssay Description:Positive allosteric modulation of human GluA2 receptor flop isoform assessed as increase in glutamate-induced calcium flux measured at time interval ...More data for this Ligand-Target Pair