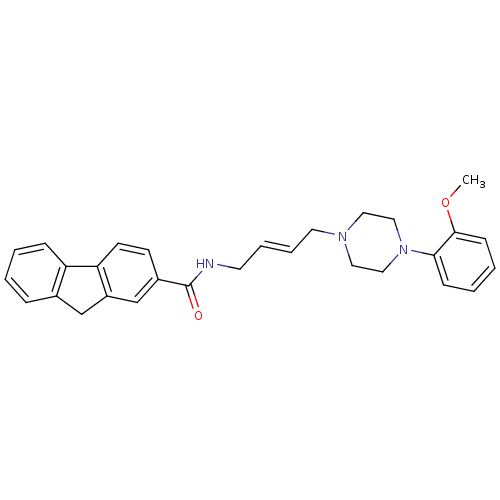
BDBM50219113 CHEMBL244989::N-(4-(4-(2-methoxyphenyl)piperazin-1-yl)-trans-but-2-enyl)-9H-fluorene-2-carboxamide::US8748608, 15
SMILES COc1ccccc1N1CCN(C\C=C\CNC(=O)c2ccc-3c(Cc4ccccc-34)c2)CC1
InChI Key InChIKey=UUYDEYACQFNFMI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50219113
Found 9 hits for monomerid = 50219113
TargetD(3) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Displacement of [125I]IABN from human dopamine D3 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 12nM IC50: 18.9nMAssay Description:Methods for performing in vitro dopamine receptor binding studies are described in Huang et al. J. Med. Chem. 44:1815-1826 (2001) and Luedtke et al. ...More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataIC50: 18.9nMAssay Description:Antagonist activity at human dopamine D3 receptor expressed in HEK293 cells by mitogenesis assayMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 48.4nMAssay Description:Displacement of [125I]IABN from human dopamine D2 receptor expressed in HEK293 cellsMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 65.7nMAssay Description:Binding affinity to human 5HT2A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 71.8nMAssay Description:Binding affinity to human 5HT1A receptorMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 176nMAssay Description:Binding affinity to human 5HT2C receptorMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataIC50: 179nMAssay Description:Antagonist activity at human dopamine D2 receptor expressed in HEK293 cells by mitogenesis assayMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataIC50: 179nMT: 2°CAssay Description:To measure D2 and D3 stimulation of mitogenesis (agonist assay) or D2 and D3 inhibition of quinpirole stimulation of mitogenesis (antagonist assay), ...More data for this Ligand-Target Pair